Murder on the Ovarian Express: A Tale of Non-Autonomous Cell Death in the Drosophila Ovary
- PMID: 34200604
- PMCID: PMC8228772
- DOI: 10.3390/cells10061454
Murder on the Ovarian Express: A Tale of Non-Autonomous Cell Death in the Drosophila Ovary
Abstract
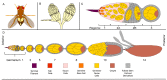
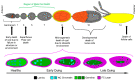
Throughout oogenesis, Drosophila egg chambers traverse the fine line between survival and death. After surviving the ten early and middle stages of oogenesis, egg chambers drastically change their size and structure to produce fully developed oocytes. The development of an oocyte comes at a cost, the price is the lives of the oocyte's 15 siblings, the nurse cells. These nurse cells do not die of their own accord. Their death is dependent upon their neighbors-the stretch follicle cells. Stretch follicle cells are nonprofessional phagocytes that spend the final stages of oogenesis surrounding the nurse cells and subsequently forcing the nurse cells to give up everything for the sake of the oocyte. In this review, we provide an overview of cell death in the ovary, with a focus on recent findings concerning this phagocyte-dependent non-autonomous cell death.
Keywords: Drosophila; cell corpse clearance; efferocytosis; oogenesis; ovary; phagocytosis; phagoptosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Lockshin R.A., Williams C.M. Programmed cell death-II. Endocrine potentiation of the breakdown of the intersegmental muscles of silkmoths. J. Insect Physiol. 1964;10:643–649. doi: 10.1016/0022-1910(64)90034-4. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

