Role of Vesicle-Associated Membrane Protein-Associated Proteins (VAP) A and VAPB in Nuclear Egress of the Alphaherpesvirus Pseudorabies Virus
- PMID: 34200728
- PMCID: PMC8229525
- DOI: 10.3390/v13061117
Role of Vesicle-Associated Membrane Protein-Associated Proteins (VAP) A and VAPB in Nuclear Egress of the Alphaherpesvirus Pseudorabies Virus
Abstract
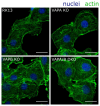
The molecular mechanism affecting translocation of newly synthesized herpesvirus nucleocapsids from the nucleus into the cytoplasm is still not fully understood. The viral nuclear egress complex (NEC) mediates budding at and scission from the inner nuclear membrane, but the NEC is not sufficient for efficient fusion of the primary virion envelope with the outer nuclear membrane. Since no other viral protein was found to be essential for this process, it was suggested that a cellular machinery is recruited by viral proteins. However, knowledge on fusion mechanisms involving the nuclear membranes is rare. Recently, vesicle-associated membrane protein-associated protein B (VAPB) was shown to play a role in nuclear egress of herpes simplex virus 1 (HSV-1). To test this for the related alphaherpesvirus pseudorabies virus (PrV), we mutated genes encoding VAPB and VAPA by CRISPR/Cas9-based genome editing in our standard rabbit kidney cells (RK13), either individually or in combination. Single as well as double knockout cells were tested for virus propagation and for defects in nuclear egress. However, no deficiency in virus replication nor any effect on nuclear egress was obvious suggesting that VAPB and VAPA do not play a significant role in this process during PrV infection in RK13 cells.
Keywords: CRISPR/Cas9 genome editing; PrV; VAPA; VAPB; herpesvirus; nuclear egress; pseudorabies virus; vesicle-associated membrane protein associated protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


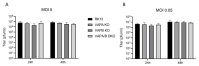

Similar articles
-
Host Vesicle Fusion Protein VAPB Contributes to the Nuclear Egress Stage of Herpes Simplex Virus Type-1 (HSV-1) Replication.Cells. 2019 Feb 3;8(2):120. doi: 10.3390/cells8020120. Cells. 2019. PMID: 30717447 Free PMC article.
-
Mutational Functional Analysis of the Pseudorabies Virus Nuclear Egress Complex-Nucleocapsid Interaction.J Virol. 2020 Mar 31;94(8):e01910-19. doi: 10.1128/JVI.01910-19. Print 2020 Mar 31. J Virol. 2020. PMID: 32051272 Free PMC article.
-
Generation and characterization of monoclonal antibodies specific for the Pseudorabies Virus nuclear egress complex.Virus Res. 2020 Oct 2;287:198096. doi: 10.1016/j.virusres.2020.198096. Epub 2020 Jul 17. Virus Res. 2020. PMID: 32682818
-
The Knowns and Unknowns of Herpesvirus Nuclear Egress.Annu Rev Virol. 2023 Sep 29;10(1):305-323. doi: 10.1146/annurev-virology-111821-105518. Epub 2023 Apr 11. Annu Rev Virol. 2023. PMID: 37040797 Review.
-
Host and Viral Factors Involved in Nuclear Egress of Herpes Simplex Virus 1.Viruses. 2021 Apr 25;13(5):754. doi: 10.3390/v13050754. Viruses. 2021. PMID: 33923040 Free PMC article. Review.
Cited by
-
Phosphorylation of ELYS promotes its interaction with VAPB at decondensing chromosomes during mitosis.EMBO Rep. 2024 May;25(5):2391-2417. doi: 10.1038/s44319-024-00125-6. Epub 2024 Apr 11. EMBO Rep. 2024. PMID: 38605278 Free PMC article.
References
-
- Fields B.K.D. Fields Virology. Volume 1 Wolters Kluwer Health; Philadelphia, PA, USA: 2007.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

