Structure, Biosynthesis, and Biological Activity of Succinylated Forms of Bacteriocin BacSp222
- PMID: 34200765
- PMCID: PMC8230399
- DOI: 10.3390/ijms22126256
Structure, Biosynthesis, and Biological Activity of Succinylated Forms of Bacteriocin BacSp222
Abstract
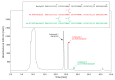
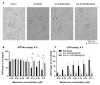
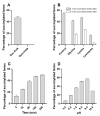
BacSp222 is a multifunctional peptide produced by Staphylococcus pseudintermedius 222. This 50-amino acid long peptide belongs to subclass IId of bacteriocins and forms a four-helix bundle molecule. In addition to bactericidal functions, BacSp222 possesses also features of a virulence factor, manifested in immunomodulatory and cytotoxic activities toward eukaryotic cells. In the present study, we demonstrate that BacSp222 is produced in several post-translationally modified forms, succinylated at the ε-amino group of lysine residues. Such modifications have not been previously described for any bacteriocins. NMR and circular dichroism spectroscopy studies have shown that the modifications do not alter the spatial structure of the peptide. At the same time, succinylation significantly diminishes its bactericidal and cytotoxic potential. We demonstrate that the modification of the bacteriocin is an effect of non-enzymatic reaction with a highly reactive intracellular metabolite, i.e., succinyl-coenzyme A. The production of succinylated forms of the bacteriocin depends on environmental factors and on the access of bacteria to nutrients. Our study indicates that the production of succinylated forms of bacteriocin occurs in response to the changing environment, protects producer cells against the autotoxicity of the excreted peptide, and limits the pathogenicity of the strain.
Keywords: Krebs/tricarboxylic acids cycle; Staphylococcus pseudintermedius; antibacterial; bacteriocin; cytotoxic; nuclear magnetic resonance (NMR); succinyl-coenzyme A; succinylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Bacteriocin BacSp222 and Its Succinylated Forms Exhibit Proinflammatory Activities Toward Innate Immune Cells .J Inflamm Res. 2022 Aug 12;15:4601-4621. doi: 10.2147/JIR.S362066. eCollection 2022. J Inflamm Res. 2022. PMID: 35982757 Free PMC article.
-
Spatial attributes of the four-helix bundle group of bacteriocins - The high-resolution structure of BacSp222 in solution.Int J Biol Macromol. 2018 Feb;107(Pt B):2715-2724. doi: 10.1016/j.ijbiomac.2017.10.158. Epub 2017 Oct 31. Int J Biol Macromol. 2018. PMID: 29107139
-
A peptide factor secreted by Staphylococcus pseudintermedius exhibits properties of both bacteriocins and virulence factors.Sci Rep. 2015 Sep 28;5:14569. doi: 10.1038/srep14569. Sci Rep. 2015. PMID: 26411997 Free PMC article.
-
Peptide AS-48: prototype of a new class of cyclic bacteriocins.Curr Protein Pept Sci. 2004 Oct;5(5):399-416. doi: 10.2174/1389203043379567. Curr Protein Pept Sci. 2004. PMID: 15544535 Review.
-
The two-peptide class II bacteriocins: structure, production, and mode of action.J Mol Microbiol Biotechnol. 2007;13(4):210-9. doi: 10.1159/000104750. J Mol Microbiol Biotechnol. 2007. PMID: 17827971 Review.
Cited by
-
Bacteriocin BacSp222 and Its Succinylated Forms Exhibit Proinflammatory Activities Toward Innate Immune Cells .J Inflamm Res. 2022 Aug 12;15:4601-4621. doi: 10.2147/JIR.S362066. eCollection 2022. J Inflamm Res. 2022. PMID: 35982757 Free PMC article.
-
Aeromonas allosaccharophila Strain AE59-TE2 Is Highly Antagonistic towards Multidrug-Resistant Human Pathogens, What Does Its Genome Tell Us?Life (Basel). 2022 Sep 26;12(10):1492. doi: 10.3390/life12101492. Life (Basel). 2022. PMID: 36294926 Free PMC article.
-
Interactions between Lipopolysaccharide and Peptide Bacteriocin BacSp222 Influence Their Biological Activities.ACS Infect Dis. 2025 Aug 8;11(8):2116-2130. doi: 10.1021/acsinfecdis.5c00066. Epub 2025 Jul 8. ACS Infect Dis. 2025. PMID: 40625076 Free PMC article.
-
BacSp222 bacteriocin as a novel ligand for TLR2/TLR6 heterodimer.Inflamm Res. 2023 May;72(5):915-928. doi: 10.1007/s00011-023-01721-3. Epub 2023 Mar 25. Inflamm Res. 2023. PMID: 36964784 Free PMC article.
-
Immunomodulation, Bioavailability and Safety of Bacteriocins.Life (Basel). 2023 Jul 7;13(7):1521. doi: 10.3390/life13071521. Life (Basel). 2023. PMID: 37511896 Free PMC article. Review.
References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

