New Perspectives on the Biogenesis of Viral Inclusion Bodies in Negative-Sense RNA Virus Infections
- PMID: 34200781
- PMCID: PMC8230417
- DOI: 10.3390/cells10061460
New Perspectives on the Biogenesis of Viral Inclusion Bodies in Negative-Sense RNA Virus Infections
Abstract
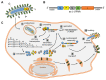
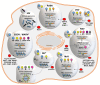
Infections by negative strand RNA viruses (NSVs) induce the formation of viral inclusion bodies (IBs) in the host cell that segregate viral as well as cellular proteins to enable efficient viral replication. The induction of those membrane-less viral compartments leads inevitably to structural remodeling of the cellular architecture. Recent studies suggested that viral IBs have properties of biomolecular condensates (or liquid organelles), as have previously been shown for other membrane-less cellular compartments like stress granules or P-bodies. Biomolecular condensates are highly dynamic structures formed by liquid-liquid phase separation (LLPS). Key drivers for LLPS in cells are multivalent protein:protein and protein:RNA interactions leading to specialized areas in the cell that recruit molecules with similar properties, while other non-similar molecules are excluded. These typical features of cellular biomolecular condensates are also a common characteristic in the biogenesis of viral inclusion bodies. Viral IBs are predominantly induced by the expression of the viral nucleoprotein (N, NP) and phosphoprotein (P); both are characterized by a special protein architecture containing multiple disordered regions and RNA-binding domains that contribute to different protein functions. P keeps N soluble after expression to allow a concerted binding of N to the viral RNA. This results in the encapsidation of the viral genome by N, while P acts additionally as a cofactor for the viral polymerase, enabling viral transcription and replication. Here, we will review the formation and function of those viral inclusion bodies upon infection with NSVs with respect to their nature as biomolecular condensates.
Keywords: biomolecular condensates; liquid-liquid phase separation (LLPS); negative strand RNA viruses (NSV); nucleoprotein; phosphoprotein; viral inclusion bodies; viral replication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Romero-Brey I., Merz A., Chiramel A., Lee J.-Y., Chlanda P., Haselman U., Santarella-Mellwig R., Habermann A., Hoppe S., Kallis S., et al. Three-dimensional architecture and biogenesis of membrane structures associated with hepatitis C virus replication. PLoS Pathog. 2012;8:e1003056. doi: 10.1371/journal.ppat.1003056. - DOI - PMC - PubMed
-
- Romero-Brey I., Berger C., Kallis S., Kolovou A., Paul D., Lohmann V., Bartenschlager R. NS5A Domain 1 and Polyprotein Cleavage Kinetics Are Critical for Induction of Double-Membrane Vesicles Associated with Hepatitis C Virus Replication. mBio. 2015;6:e00759. doi: 10.1128/mBio.00759-15. - DOI - PMC - PubMed
-
- Welsch S., Miller S., Romero-Brey I., Merz A., Bleck C.K.E., Walther P., Fuller S.D., Antony C., Krijnse-Locker J., Bartenschlager R. Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell Host Microbe. 2009;5:365–375. doi: 10.1016/j.chom.2009.03.007. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

