Inhibition of Human Malignant Pleural Mesothelioma Growth by Mesenchymal Stromal Cells
- PMID: 34201002
- PMCID: PMC8227879
- DOI: 10.3390/cells10061427
Inhibition of Human Malignant Pleural Mesothelioma Growth by Mesenchymal Stromal Cells
Abstract
Background: Malignant Pleural Mesothelioma (MPM) is an aggressive tumor that has a significant incidence related to asbestos exposure with no effective therapy and poor prognosis. The role of mesenchymal stromal cells (MSCs) in cancer is controversial due to their opposite effects on tumor growth and in particular, only a few data are reported on MSCs and MPM.
Methods: We investigated the in vitro efficacy of adipose tissue-derived MSCs, their lysates and secretome against different MPM cell lines. After large-scale production of MSCs in a bioreactor, their efficacy was also evaluated on a human MPM xenograft in mice.
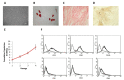
Results: MSCs, their lysate and secretome inhibited MPM cell proliferation in vitro with S or G0/G1 arrest of the cell cycle, respectively. MSC lysate induced cell death by apoptosis. The efficacy of MSC was confirmed in vivo by a significant inhibition of tumor growth, similar to that produced by systemic administration of paclitaxel. Interestingly, no tumor progression was observed after the last MSC treatment, while tumors started to grow again after stopping chemotherapeutic treatment.
Conclusions: These data demonstrated for the first time that MSCs, both through paracrine and cell-to-cell interaction mechanisms, induced a significant inhibition of human mesothelioma growth. Since the prognosis for MPM patients is poor and the options of care are limited to chemotherapy, MSCs could provide a potential new therapeutic approach for this malignancy.
Keywords: cell therapy; malignant pleural mesothelioma (MPM); mesenchymal stromal cells; mesothelioma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Krug L.M., Pass H.I., Rusch V.W., Kindler H.L., Sugarbaker D.J., Rosenzweig K.E., Flores R., Friedberg J.S., Pisters K., Monberg M., et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J. Clin. Oncol. 2009;27:3007–3013. doi: 10.1200/JCO.2008.20.3943. - DOI - PMC - PubMed

