The Combined Human Genotype of Truncating TTN and RBM20 Mutations Is Associated with Severe and Early Onset of Dilated Cardiomyopathy
- PMID: 34201072
- PMCID: PMC8228627
- DOI: 10.3390/genes12060883
The Combined Human Genotype of Truncating TTN and RBM20 Mutations Is Associated with Severe and Early Onset of Dilated Cardiomyopathy
Abstract
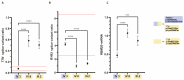
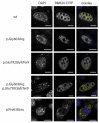
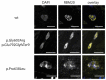
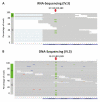
A major cause of heart failure is cardiomyopathies, with dilated cardiomyopathy (DCM) as the most common form. Over 40 genes are linked to DCM, among them TTN and RBM20. Next Generation Sequencing in clinical DCM cohorts revealed truncating variants in TTN (TTNtv), accounting for up to 25% of familial DCM cases. Mutations in the cardiac splicing factor RNA binding motif protein 20 (RBM20) are also known to be associated with severe cardiomyopathies. TTN is one of the major RBM20 splicing targets. Most of the pathogenic RBM20 mutations are localized in the highly conserved arginine serine rich domain (RS), leading to a cytoplasmic mislocalization of mutant RBM20. Here, we present a patient with an early onset DCM carrying a combination of (likely) pathogenic TTN and RBM20 mutations. We show that the splicing of RBM20 target genes is affected in the mutation carrier. Furthermore, we reveal RBM20 haploinsufficiency presumably caused by the frameshift mutation in RBM20.
Keywords: RBM20; TTN; cardiomyopathy; haploinsufficiency; mutation.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures

References
-
- Khush K.K., Cherikh W.S., Chambers D.C., Goldfarb S., Hayes D., Jr., Kucheryavaya A.Y., Levvey B.J., Meiser B., Rossano J.W., Stehlik J., et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth Adult Heart Transplantation Report-2018; Focus Theme: Multiorgan Transplantation. J. Heart Lung Transpl. 2018;37:1155–1168. doi: 10.1016/j.healun.2018.07.022. - DOI - PubMed
-
- Rossano J.W., Cherikh W.S., Chambers D.C., Goldfarb S., Hayes D., Jr., Khush K.K., Kucheryavaya A.Y., Toll A.E., Levvey B.J., Meiser B., et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Twenty-first pediatric heart transplantation report-2018; Focus theme: Multiorgan Transplantation. J. Heart Lung Transpl. 2018;37:1184–1195. doi: 10.1016/j.healun.2018.07.018. - DOI - PubMed
-
- McMurray J.J., Adamopoulos S., Anker S.D., Auricchio A., Bohm M., Dickstein K., Falk V., Filippatos G., Fonseca C., Gomez-Sanchez M.A., et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

