NQS-Doped PDMS Solid Sensor: From Water Matrix to Urine Enzymatic Application
- PMID: 34201174
- PMCID: PMC8228043
- DOI: 10.3390/bios11060186
NQS-Doped PDMS Solid Sensor: From Water Matrix to Urine Enzymatic Application
Abstract
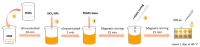
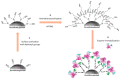
The development of in situ analytical devices has gained outstanding scientific interest. A solid sensing membrane composed of 1,2-naphthoquinone-4-sulfonate (NQS) derivatizing reagent embedded into a polymeric polydimethylsiloxane (PDMS) composite was proposed for in situ ammonium (NH4+) and urea (NH2CONH2) analysis in water and urine samples, respectively. Satisfactory strategies were also applied for urease-catalyzed hydrolysis of urea, either in solution or glass-supported urease immobilization. Using diffuse reflectance measurements combined with digital image processing of color intensity (RGB coordinates), qualitative and quantitative analyte detection was assessed after the colorimetric reaction took place inside the sensing membrane. A suitable linear relationship was found between the sensor response and analyte concentration, and the results were validated by a thymol-PDMS-based sensor based on the Berthelot reaction. The suggested sensing device offers advantages such as rapidity, versatility, portability, and employment of non-toxic reagents that facilitate in situ analysis in an energy-efficient manner.
Keywords: NQS-PDMS sensor; ammonium; glass support; in-situ analysis; optical sensor; urea; urea hydrolysis; urease; urine; water.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Development of a polydimethylsiloxane-thymol/nitroprusside composite based sensor involving thymol derivatization for ammonium monitoring in water samples.Sci Total Environ. 2015 Jan 15;503-504:105-12. doi: 10.1016/j.scitotenv.2014.07.077. Epub 2014 Aug 10. Sci Total Environ. 2015. PMID: 25113949
-
A solid device based on doped hybrid composites for controlling the dosage of the biocide N-(3-aminopropyl)-N-dodecyl-1,3-propanediamine in industrial formulations.Talanta. 2016 Jan 15;147:147-54. doi: 10.1016/j.talanta.2015.09.051. Epub 2015 Sep 25. Talanta. 2016. PMID: 26592589
-
Polydimethylsiloxane composites containing 1,2-naphtoquinone 4-sulphonate as unique dispositive for estimation of casein in effluents from dairy industries.Anal Chim Acta. 2015 May 11;873:31-7. doi: 10.1016/j.aca.2015.02.057. Epub 2015 Feb 23. Anal Chim Acta. 2015. PMID: 25911427
-
Ionic-liquid doped polymeric composite as passive colorimetric sensor for meat freshness as a use case.Talanta. 2021 Feb 1;223(Pt 2):121778. doi: 10.1016/j.talanta.2020.121778. Epub 2020 Oct 15. Talanta. 2021. PMID: 33298283
-
1,2-Naphthoquinone-4-sulfonic acid salts in organic synthesis.Beilstein J Org Chem. 2022 Jan 5;18:53-69. doi: 10.3762/bjoc.18.5. eCollection 2022. Beilstein J Org Chem. 2022. PMID: 35047082 Free PMC article. Review.
Cited by
-
A Hybrid Titanium-Softmaterial, High-Strength, Transparent Cranial Window for Transcranial Injection and Neuroimaging.Biosensors (Basel). 2022 Feb 18;12(2):129. doi: 10.3390/bios12020129. Biosensors (Basel). 2022. PMID: 35200389 Free PMC article.
-
Advances in the Measurement of Polymeric Colorimetric Sensors Using Portable Instrumentation: Testing the Light Influence.Polymers (Basel). 2022 Oct 12;14(20):4285. doi: 10.3390/polym14204285. Polymers (Basel). 2022. PMID: 36297863 Free PMC article.
References
-
- Ni J.-Q., Diehl C.A., Chai L., Chen Y., Heber A.J., Lim T.-T., Bogan B.W. Factors and characteristics of ammonia, hydrogen sulfide, carbon dioxide, and particulate matter emissions from two manure-belt layer hen houses. Atmos. Environ. 2017;156:113–124. doi: 10.1016/j.atmosenv.2017.02.033. - DOI
-
- Williams Ischer S., Farnell M.B., Tabler G.T., Moreira M., O’Shaughnessy P.T., Nonnenmann M.W. Evaluation of a sprinkler cooling system on inhalable dust and ammonia concentrations in broiler chicken production. J. Occup. Environ. Hyg. 2017;14:40–48. doi: 10.1080/15459624.2016.1211285. - DOI - PMC - PubMed
-
- Zhao L., Hadlocon L.J.S., Manuzon R.B., Darr M.J., Keener H.M., Heber A.J., Ni J. Ammonia concentrations and emission rates at a commercial poultry manure composting facility. Biosyst. Eng. 2016;150:69–78. doi: 10.1016/j.biosystemseng.2016.07.006. - DOI
-
- Li D., Watson C.J., Yan M.J., Lalor S., Rafique R., Hyde B., Lanigan G., Richards K.G., Holden N.M., Humphreys J. A review of nitrous oxide mitigation by farm nitrogen management in temperate grassland-based agriculture. J. Environ. Manag. 2013;128:893–903. doi: 10.1016/j.jenvman.2013.06.026. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

