T-Type Ca2+ Enhancer SAK3 Activates CaMKII and Proteasome Activities in Lewy Body Dementia Mice Model
- PMID: 34201181
- PMCID: PMC8228122
- DOI: 10.3390/ijms22126185
T-Type Ca2+ Enhancer SAK3 Activates CaMKII and Proteasome Activities in Lewy Body Dementia Mice Model
Abstract
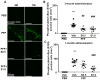
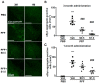
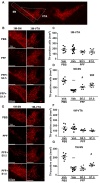
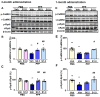
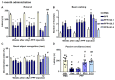
Lewy bodies are pathological characteristics of Lewy body dementia (LBD) and are composed of α-synuclein (α-Syn), which is mostly degraded via the ubiquitin-proteasome system. More importantly, 26S proteasomal activity decreases in the brain of LBD patients. We recently introduced a T-type calcium channel enhancer SAK3 (ethyl-8-methyl-2,4-dioxo-2-(piperidin-1-yl)- 2H-spiro[cyclopentane-1,3-imidazo [1,2-a]pyridin]-2-ene-3-carboxylate) for Alzheimer's disease therapeutics. SAK3 enhanced the proteasome activity via CaMKII activation in amyloid precursor protein knock-in mice, promoting the degradation of amyloid-β plaques to improve cognition. At this point, we addressed whether SAK3 promotes the degradation of misfolded α-Syn and the aggregates in α-Syn preformed fibril (PFF)-injected mice. The mice were injected with α-Syn PFF in the dorsal striatum, and SAK3 (0.5 or 1.0 mg/kg) was administered orally for three months, either immediately or during the last month after injection. SAK3 significantly inhibited the accumulation of fibrilized phosphorylated-α-Syn in the substantia nigra. Accordingly, SAK3 significantly recovered mesencephalic dopamine neurons from cell death. Decreased α-Syn accumulation was closely associated with increased proteasome activity. Elevated CaMKII/Rpt-6 signaling possibly mediates the enhanced proteasome activity after SAK3 administration in the cortex and hippocampus. CaMKII/Rpt-6 activation also accounted for improved memory and cognition in α-Syn PFF-injected mice. These findings indicate that CaMKII/Rpt-6-dependent proteasomal activation by SAK3 recovers from α-Syn pathology in LBD.
Keywords: Alzheimer’s disease; Lewy body dementia; SAK3; T-type Ca2+ channel enhancer; alpha-synuclein; amyloid β plaque; proteasome activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

