Development of a Simple and Robust Whole Blood Assay with Dual Co-Stimulation to Quantify the Release of T-Cellular Signature Cytokines in Response to Aspergillus fumigatus Antigens
- PMID: 34201183
- PMCID: PMC8230040
- DOI: 10.3390/jof7060462
Development of a Simple and Robust Whole Blood Assay with Dual Co-Stimulation to Quantify the Release of T-Cellular Signature Cytokines in Response to Aspergillus fumigatus Antigens
Abstract
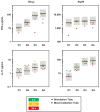
Deeper understanding of mold-induced cytokine signatures could promote advances in the diagnosis and treatment of invasive mycoses and mold-associated hypersensitivity syndromes. Currently, most T-cellular immunoassays in medical mycology require the isolation of mononuclear cells and have limited robustness and practicability, hampering their broader applicability in clinical practice. Therefore, we developed a simple, cost-efficient whole blood (WB) assay with dual α-CD28 and α-CD49d co-stimulation to quantify cytokine secretion in response to Aspergillus fumigatus antigens. Dual co-stimulation strongly enhanced A. fumigatus-induced release of T-cellular signature cytokines detectable by enzyme-linked immunosorbent assay (ELISA) or a multiplex cytokine assay. Furthermore, T-cell-dependent activation and cytokine response of innate immune cells was captured by the assay. The protocol consistently showed little technical variation and high robustness to pre-analytic delays of up to 8 h. Stimulation with an A. fumigatus lysate elicited at least 7-fold greater median concentrations of key T-helper cell signature cytokines, including IL-17 and the type 2 T-helper cell cytokines IL-4 and IL-5 in WB samples from patients with Aspergillus-associated lung pathologies versus patients with non-mold-related lung diseases, suggesting high discriminatory power of the assay. These results position WB-ELISA with dual co-stimulation as a simple, accurate, and robust immunoassay for translational applications, encouraging further evaluation as a platform to monitor host immunity to opportunistic pathogens.
Keywords: Aspergillus; adaptive immunity; biomarker; cytokines; immunoassay; inflammation.
Conflict of interest statement
The authors have no conflicts of interest related to this study.
Figures




References
-
- Wurster S., Weis P., Page L., Helm J., Lazariotou M., Einsele H., Ullmann A.J. Intra- and inter-individual variability of Aspergillus fumigatus reactive T-cell frequencies in healthy volunteers in dependency of mould exposure in residential and working environment. Mycoses. 2017;60:668–675. doi: 10.1111/myc.12643. - DOI - PubMed
-
- Jolink H., Hagedoorn R.S., Lagendijk E.L., Drijfhout J.W., van Dissel J.T., Falkenburg J.H., Heemskerk M.H. Induction of A. fumigatus-specific CD4-positive T cells in patients recovering from invasive aspergillosis. Haematologica. 2014;99:1255–1263. doi: 10.3324/haematol.2013.098830. - DOI - PMC - PubMed
-
- Lordan J.L., Bucchieri F., Richter A., Konstantinidis A., Holloway J.W., Thornber M., Puddicombe S.M., Buchanan D., Wilson S.J., Djukanovic R., et al. Cooperative effects of Th2 cytokines and allergen on normal and asthmatic bronchial epithelial cells. J. Immunol. 2002;169:407–414. doi: 10.4049/jimmunol.169.1.407. - DOI - PubMed
LinkOut - more resources
Full Text Sources

