Ocular Graft-versus-Host Disease in a Chemotherapy-Based Minor-Mismatch Mouse Model Features Corneal (Lymph-) Angiogenesis
- PMID: 34201218
- PMCID: PMC8228997
- DOI: 10.3390/ijms22126191
Ocular Graft-versus-Host Disease in a Chemotherapy-Based Minor-Mismatch Mouse Model Features Corneal (Lymph-) Angiogenesis
Abstract
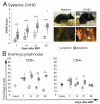
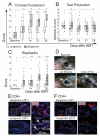
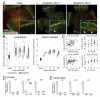
Ocular graft-versus-host disease (oGVHD) is a fast progressing, autoimmunological disease following hematopoietic stem cell transplantation, leading to severe inflammation of the eye and destruction of the lacrimal functional unit with consecutive sight-threatening consequences. The therapeutic "window of opportunity" is narrow, and current treatment options are limited and often insufficient. To achieve new insights into the pathogenesis and to develop new therapeutic approaches, clinically relevant models of oGVHD are desirable. In this study, the ocular phenotype was described in a murine, chemotherapy-based, minor-mismatch GVHD model mimicking early-onset chronic oGVHD, with corneal epitheliopathy, inflammation of the lacrimal glands, and blepharitis. Additionally, corneal lymphangiogenesis was observed as part of oGVHD pathogenesis for the first time, thus opening up the investigation of lymphangiogenesis as a potential therapeutic and diagnostic tool.
Keywords: blepharitis; chemotherapy; lymphangiogenesis; oGVHD; ocular graft-versus-host-disease; pre-clinical model.
Conflict of interest statement
D.S., M.M., K.R., G.M., O.P., and U.G. declare no conflict of interest. M.E.S. is the chief scientific officer of ImmunEyez LLC and a member of the scientific advisory board of Novaliq. P.S. has received financial support from Novaliq GmbH, Roche, Bausch & Lomb, and Ursapharm. The Division of dry-eye and ocular GVHD received donations from Novaliq, Ursapharm, and Juergen and Monika Ziehm.
Figures

References
-
- Ogawa Y., Kim S.K., Dana R., Clayton J., Jain S., Rosenblatt M.I., Perez V.L., Shikari H., Riemens A., Tsubota K. International Chronic Ocular Graft-vs-Host-Disease (GVHD) Consensus Group: Proposed diagnostic criteria for chronic GVHD (Part I) Sci. Rep. 2013;3:3419. doi: 10.1038/srep03419. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

