Histone Deacetylase Inhibitors Increase the Embryogenic Potential and Alter the Expression of Embryogenesis-Related and HDAC-Encoding Genes in Grapevine (Vitis vinifera L., cv. Mencía)
- PMID: 34201224
- PMCID: PMC8228518
- DOI: 10.3390/plants10061164
Histone Deacetylase Inhibitors Increase the Embryogenic Potential and Alter the Expression of Embryogenesis-Related and HDAC-Encoding Genes in Grapevine (Vitis vinifera L., cv. Mencía)
Abstract
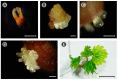
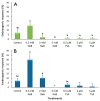
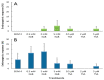
The low induction rates of somatic embryogenesis are one of the main limitations in its routine application in the grapevine (Vitis vinifera L.). The use of an induction medium containing histone deacetylase inhibitors (trichostatin A and, mainly, sodium butyrate) resulted in an improvement of the embryogenic responses in grapevine (cv. Mencía) cotyledonary and recently germinated somatic embryos. The relative expression of several grapevine genes related to embryogenic competence or encoding histone deacetylase enzymes was studied in cotyledonary somatic embryos that were cultured in the presence of 0.5 mM sodium butyrate. The results showed a significant overexpression of the BBM and VvSERK2 genes after 24 h of culture, whereas the VvWOX2 gene was underexpressed less in treated versus untreated explants. The results suggest that the inhibitor may trigger a molecular response related to an increase in embryogenic competence and changes in the expression of associated genes. The treatment with sodium butyrate also produced significant variations in the expression of several histone deacetylase enzyme-encoding genes. These results may enhance the possibility of obtaining somatic embryos, reducing the seasonal constraints associated with the use of floral explants in grapevines.
Keywords: BABY BOOM; SERK2; Vitis vinifera; gene expression; histone deacetylase-encoding genes; sodium butyrate; somatic embryogenesis; trichostatin A.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Changes in abscisic acid metabolism in relation to the maturation of grapevine (Vitis vinifera L., cv. Mencía) somatic embryos.BMC Plant Biol. 2020 Oct 23;20(1):487. doi: 10.1186/s12870-020-02701-z. BMC Plant Biol. 2020. PMID: 33097003 Free PMC article.
-
Characterization of VvSERK1, VvSERK2, VvSERK3 and VvL1L genes and their expression during somatic embryogenesis of grapevine (Vitis vinifera L.).Plant Cell Rep. 2008 Dec;27(12):1799-809. doi: 10.1007/s00299-008-0588-8. Epub 2008 Sep 3. Plant Cell Rep. 2008. PMID: 18766346
-
Characterization of expression dynamics of WOX homeodomain transcription factors during somatic embryogenesis in Vitis vinifera.J Exp Bot. 2011 Jan;62(3):1089-101. doi: 10.1093/jxb/erq349. Epub 2010 Dec 1. J Exp Bot. 2011. PMID: 21127025
-
Development and Applications of Somatic Embryogenesis in Grapevine (Vitis spp.).Plants (Basel). 2024 Nov 7;13(22):3131. doi: 10.3390/plants13223131. Plants (Basel). 2024. PMID: 39599340 Free PMC article. Review.
-
[Epigenetic mechanisms and alcohol use disorders: a potential therapeutic target].Biol Aujourdhui. 2017;211(1):83-91. doi: 10.1051/jbio/2017014. Epub 2017 Jul 6. Biol Aujourdhui. 2017. PMID: 28682229 Review. French.
Cited by
-
Small molecules, enormous functions: potential approach for overcoming bottlenecks in embryogenic tissue induction and maintenance in conifers.Hortic Res. 2024 Jul 10;11(8):uhae180. doi: 10.1093/hr/uhae180. eCollection 2024 Aug. Hortic Res. 2024. PMID: 39108576 Free PMC article. Review.
-
Effects of TSA, NaB, Aza in Lactuca sativa L. protoplasts and effect of TSA in Nicotiana benthamiana protoplasts on cell division and callus formation.PLoS One. 2023 Feb 24;18(2):e0279627. doi: 10.1371/journal.pone.0279627. eCollection 2023. PLoS One. 2023. PMID: 36827385 Free PMC article.
-
Osmotic priming-induced cryotolerance uncovers rejuvenation of grapevine cell cultures: morphogenetic changes and gene expression pattern highlighting enhanced embryogenic potential.Protoplasma. 2024 Nov;261(6):1251-1266. doi: 10.1007/s00709-024-01968-5. Epub 2024 Jul 9. Protoplasma. 2024. PMID: 38980351
-
Insights into the Histone Acetylation-Mediated Regulation of the Transcription Factor Genes That Control the Embryogenic Transition in the Somatic Cells of Arabidopsis.Cells. 2022 Mar 2;11(5):863. doi: 10.3390/cells11050863. Cells. 2022. PMID: 35269485 Free PMC article.
-
Histone Deacetylase Inhibitor, Sodium Butyrate-Induced Metabolic Modulation in Platycodon grandiflorus Roots Enhances Anti-Melanogenic Properties.Int J Mol Sci. 2023 Jul 22;24(14):11804. doi: 10.3390/ijms241411804. Int J Mol Sci. 2023. PMID: 37511563 Free PMC article.
References
-
- Yang X., Zhang X. Regulation of somatic embryogenesis in higher plants. CRC Crit. Rev. Plant Sci. 2010;29:36–57. doi: 10.1080/07352680903436291. - DOI
-
- Dhekney S.A., Basford A.T., Chhatre V.E., Rosenberg M.B., Claflin C., Sessions S.K., Li Z.J., Gray D.J. Vitis spp. Grape. In: Litz R.E., Pliego-Alfaro F., Hormaza J.I., editors. Biotechnology of Fruit and Nut Crops. 2nd ed. CAB International; Boston, MA, USA: 2020. pp. 655–674.
-
- Martinelli L., Gribaudo I. Strategies for effective somatic embryogenesis in grapevine: An appraisal. In: Roubelakis-Angelakis K.A., editor. Grapevine Molecular Physiology & Biotechnology. Springer; Dordrecht, The Netherlands: 2009. pp. 461–493. - DOI
-
- Gribaudo I., Gambino G., Vallania R. Somatic embryogenesis from grapevine anthers: The optimal developmental stage for collecting explants. Am. J. Enol. Vitic. 2004;55:427–430.
-
- Dhekney S.A., Li Z.T., Gray D.J. Optimizing initiation and maintenance of Vitis embryogenic cultures. HortScience. 2009;44:1400–1406. doi: 10.21273/HORTSCI.44.5.1400. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

