ctDNA to Guide Adjuvant Therapy in Localized Colorectal Cancer (CRC)
- PMID: 34201274
- PMCID: PMC8226638
- DOI: 10.3390/cancers13122869
ctDNA to Guide Adjuvant Therapy in Localized Colorectal Cancer (CRC)
Abstract
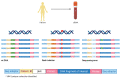
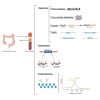
Currently, the standard treatment for patients with localized colorectal cancer (CRC) includes surgical resection followed by adjuvant chemotherapy based on clinicopathological features. Recurrence risk stratification in those patients is of utmost importance to guide clinicians to avoid both under- and overtreatment. Recently, the concept of minimal residual disease (MRD) has emerged as the detection of circulating tumor DNA (ctDNA) carrying tumor-specific genomic or epigenomic alterations in the bloodstream of patients after surgery. Emerging studies described how the detection of MRD is a powerful prognostic biomarker to identify patients at higher risk of recurrence and who will potentially benefit the most from a systemic adjuvant treatment. Based on that unprecedented finding, several clinical trials involving stage II and III CRC patients are ongoing evaluating the impact of ctDNA guided treatment by escalating or deescalating adjuvant chemotherapy based on ctDNA MRD detection. This review provides a critical overview of current perspectives of liquid biopsy in early-stage CRC including technical, biological, and clinical key points, as well as ongoing ctDNA-based clinical trials that ultimately aim to improve clinical outcomes of patients with CRC.
Keywords: cancer detection; circulating tumor DNA; colorectal cancer; liquid biopsy; minimal residual disease; next-generation sequencing.
Conflict of interest statement
J.V. received honoraria for advisory role, speaker or travel grants from Amgen, Hoffman La-Roche, Merck-Serono, Novartis, and Sanofi. C.M. received honoraria for advisory role, speaker or research grants (past 5 years): Amgen, Biocartis, Bristol Myers Squibb, Guardant-Health, Merck-Serono, Hoffman La-Roche, Sanofi Aventis. C.F.-R. received honoraria for speaker from Merck and consultancy from Roche. L.M. declares no conflict of interest.
Figures


References
-
- Argilés G., Tabernero J., Labianca R., Hochhauser D., Salazar R., Iveson T., Laurent-Puig P., Quirke P., Yoshino T., Taieb J., et al. Localised Colon Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2020:S0923753420399324. doi: 10.1016/j.annonc.2020.06.022. - DOI - PubMed
-
- Schmoll H.-J., Twelves C., Sun W., O’Connell M.J., Cartwright T., McKenna E., Saif M., Lee S., Yothers G., Haller D. Effect of Adjuvant Capecitabine or Fluorouracil, with or without Oxaliplatin, on Survival Outcomes in Stage III Colon Cancer and the Effect of Oxaliplatin on Post-Relapse Survival: A Pooled Analysis of Individual Patient Data from Four Randomised Controlled Trials. Lancet Oncol. 2014;15:1481–1492. doi: 10.1016/S1470-2045(14)70486-3. - DOI - PMC - PubMed
-
- Zaniboni A., Labianca R., Marsoni S., Torri V., Mosconi P., Grilli R., Apolone G., Cifani S., Tinazzi A. A Randomized Trial of Adjuvant 5-Fluorouracil and Folinic Acid Administered to Patients with Colon Carcinoma—Long Term Results and Evaluation of the Indicators of Health-Related Quality of Life. Cancer. 1998;82:2135–2144. doi: 10.1002/(SICI)1097-0142(19980601)82:11<2135::AID-CNCR7>3.0.CO;2-U. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

