Improper Remodeling of Organelles Deputed to Ca2+ Handling and Aerobic ATP Production Underlies Muscle Dysfunction in Ageing
- PMID: 34201319
- PMCID: PMC8228829
- DOI: 10.3390/ijms22126195
Improper Remodeling of Organelles Deputed to Ca2+ Handling and Aerobic ATP Production Underlies Muscle Dysfunction in Ageing
Abstract
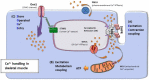
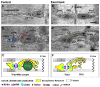
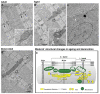
Proper skeletal muscle function is controlled by intracellular Ca2+ concentration and by efficient production of energy (ATP), which, in turn, depend on: (a) the release and re-uptake of Ca2+ from sarcoplasmic-reticulum (SR) during excitation-contraction (EC) coupling, which controls the contraction and relaxation of sarcomeres; (b) the uptake of Ca2+ into the mitochondrial matrix, which stimulates aerobic ATP production; and finally (c) the entry of Ca2+ from the extracellular space via store-operated Ca2+ entry (SOCE), a mechanism that is important to limit/delay muscle fatigue. Abnormalities in Ca2+ handling underlie many physio-pathological conditions, including dysfunction in ageing. The specific focus of this review is to discuss the importance of the proper architecture of organelles and membrane systems involved in the mechanisms introduced above for the correct skeletal muscle function. We reviewed the existing literature about EC coupling, mitochondrial Ca2+ uptake, SOCE and about the structural membranes and organelles deputed to those functions and finally, we summarized the data collected in different, but complementary, projects studying changes caused by denervation and ageing to the structure and positioning of those organelles: a. denervation of muscle fibers-an event that contributes, to some degree, to muscle loss in ageing (known as sarcopenia)-causes misplacement and damage: (i) of membrane structures involved in EC coupling (calcium release units, CRUs) and (ii) of the mitochondrial network; b. sedentary ageing causes partial disarray/damage of CRUs and of calcium entry units (CEUs, structures involved in SOCE) and loss/misplacement of mitochondria; c. functional electrical stimulation (FES) and regular exercise promote the rescue/maintenance of the proper architecture of CRUs, CEUs, and of mitochondria in both denervation and ageing. All these structural changes were accompanied by related functional changes, i.e., loss/decay in function caused by denervation and ageing, and improved function following FES or exercise. These data suggest that the integrity and proper disposition of intracellular organelles deputed to Ca2+ handling and aerobic generation of ATP is challenged by inactivity (or reduced activity); modifications in the architecture of these intracellular membrane systems may contribute to muscle dysfunction in ageing and sarcopenia.
Keywords: Ca2+ entry unit (CEU); Ca2+ release unit (CRU); excitation–contraction (EC) coupling; mitochondria; sarcoplasmic-reticulum (SR); store-operated Ca2+ entry (SOCE); transverse tubule (TT).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Mitochondria Association to Calcium Release Units is Controlled by Age and Muscle Activity.Eur J Transl Myol. 2015 Oct 27;25(4):257-62. doi: 10.4081/ejtm.2015.5604. eCollection 2015 Aug 24. Eur J Transl Myol. 2015. PMID: 26913166 Free PMC article.
-
Calcium entry units (CEUs): perspectives in skeletal muscle function and disease.J Muscle Res Cell Motil. 2021 Jun;42(2):233-249. doi: 10.1007/s10974-020-09586-3. Epub 2020 Aug 18. J Muscle Res Cell Motil. 2021. PMID: 32812118 Free PMC article.
-
Altered Ca2+ Handling and Oxidative Stress Underlie Mitochondrial Damage and Skeletal Muscle Dysfunction in Aging and Disease.Metabolites. 2021 Jun 28;11(7):424. doi: 10.3390/metabo11070424. Metabolites. 2021. PMID: 34203260 Free PMC article. Review.
-
Muscle activity prevents the uncoupling of mitochondria from Ca2+ Release Units induced by ageing and disuse.Arch Biochem Biophys. 2019 Mar 15;663:22-33. doi: 10.1016/j.abb.2018.12.017. Epub 2018 Dec 20. Arch Biochem Biophys. 2019. PMID: 30578752 Free PMC article.
-
Store-operated Ca2+ entry in muscle physiology and diseases.BMB Rep. 2014 Feb;47(2):69-79. doi: 10.5483/bmbrep.2014.47.2.015. BMB Rep. 2014. PMID: 24411466 Free PMC article. Review.
Cited by
-
Rejuvenation: Turning Back Time by Enhancing CISD2.Int J Mol Sci. 2022 Nov 13;23(22):14014. doi: 10.3390/ijms232214014. Int J Mol Sci. 2022. PMID: 36430496 Free PMC article. Review.
-
Betaine delays age-related muscle loss by mitigating Mss51-induced impairment in mitochondrial respiration via Yin Yang1.J Cachexia Sarcopenia Muscle. 2024 Oct;15(5):2104-2117. doi: 10.1002/jcsm.13558. Epub 2024 Aug 26. J Cachexia Sarcopenia Muscle. 2024. PMID: 39187977 Free PMC article.
-
2023 Padua Days of Muscle and Mobility Medicine: post-meeting Book of Abstracts.Eur J Transl Myol. 2023 Apr 27;33(2):11427. doi: 10.4081/ejtm.2023.11427. Eur J Transl Myol. 2023. PMID: 37114363 Free PMC article.
-
Constitutive, Muscle-Specific Orai1 Knockout Results in the Incomplete Assembly of Ca2+ Entry Units and a Reduction in the Age-Dependent Formation of Tubular Aggregates.Biomedicines. 2024 Jul 24;12(8):1651. doi: 10.3390/biomedicines12081651. Biomedicines. 2024. PMID: 39200116 Free PMC article.
-
Fiber-Type Shifting in Sarcopenia of Old Age: Proteomic Profiling of the Contractile Apparatus of Skeletal Muscles.Int J Mol Sci. 2023 Jan 26;24(3):2415. doi: 10.3390/ijms24032415. Int J Mol Sci. 2023. PMID: 36768735 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

