Distinct Modulatory Effects of Fever-Range Hyperthermia on the Response of Breast Cancer Cells and Macrophages to Mistletoe (Viscum album L.) Extract
- PMID: 34201348
- PMCID: PMC8229697
- DOI: 10.3390/ph14060551
Distinct Modulatory Effects of Fever-Range Hyperthermia on the Response of Breast Cancer Cells and Macrophages to Mistletoe (Viscum album L.) Extract
Abstract
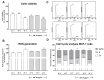
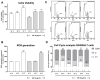
Heat utility as a critical component of fever is often ignored, although the symptom is observed in many medical conditions. Mistletoe extract (ME) is an adjunctive medication prescribed to cancer patients. The increase in body temperature is frequently observed in patients following ME administration. Nevertheless, the impact of this fever on the effectiveness of therapy is unknown. Therefore, we aimed to investigate the effect of fever-range temperatures on ME-treated breast cancer cells and macrophages. The cells were simultaneously stimulated with ME and subjected to fever-range hyperthermia (FRH; 39 °C or 41 °C). After co-treatment, the cell viability, generation of reactive oxygen species (ROS), cell cycle distribution, and production of pro-inflammatory factors (interleukin (IL)-1β, IL-6, and cyclooxygenase (COX)-2) were evaluated. The results showed that the exposure of ME-treated breast cancer cells to FRH at 39 °C resulted in a slight decrease in their viability, whereas FRH of 41 °C enhanced this effect. Only FRH of 41 °C induced minor changes in ROS level in ME-treated breast cancer cell lines. In ME-treated macrophages, FRH stimulated cell proliferation. The cell cycle distribution analysis showed a difference between cells cultured at 39 °C and 41 °C in all examined cell lines. Moreover, hyperthermia at 41 °C completely inhibited the ME-induced increase in IL-1β and IL-6 expression in MCF-7 breast cancer cells, whereas this effect was not observed in 4T1 breast cancer cells. In contrast, in ME-treated macrophages, FRH of 41 °C strongly up-regulated expression of the pro-inflammatory factors. We conclude that fever is an important component of ME therapy that differentially affects cancer and immune cells.
Keywords: cell cycle distribution; cytokines; fever; hyperthermia; inflammation; mistletoe extract; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Kienle G.S., Berrino F., Büssing A., Portalupi E., Rosenzweig S., Kiene H. Mistletoe in cancer—A systematic review on controlled clinical trials. Eur. J. Med. Res. 2003;8:109–119. - PubMed
-
- Tröger W., Galun D., Reif M., Schumann A., Stanković N., Milićević M. Viscum album [L.] extract therapy in patients with locally advanced or metastatic pancreatic cancer: A randomised clinical trial on overall survival. Eur. J. Cancer. 2013;49:3788–3797. doi: 10.1016/j.ejca.2013.06.043. - DOI - PubMed
-
- Schöffski P., Riggert S., Fumoleau P., Campone M., Bolte O., Marreaud S., Lacombe D., Baron B., Herold M., Zwierzina H., et al. Phase I trial of intravenous aviscumine (rViscumin) in patients with solid tumors: A study of the European Organization for Research and Treatment of Cancer New Drug Development Group. Ann. Oncol. 2004;15:1816–1824. doi: 10.1093/annonc/mdh469. - DOI - PubMed
Grants and funding
- POWR.03.05.00-00-Z302/17/"Universitas Copernicana Thoruniensis In Futuro" (2018-2022) co-financed from the European Social Fund - the Operational Programme Knowledge Education Development. Module 5. Inter-disciplinary PhD School "Academia Copernicana"
- No. 008/RID/2018/19/Polish Minister of Science and Higher Education under the program "Regional Initiative of Excellence" in 2019-2022
LinkOut - more resources
Full Text Sources
Research Materials

