Do Changes in ACE-2 Expression Affect SARS-CoV-2 Virulence and Related Complications: A Closer Look into Membrane-Bound and Soluble Forms
- PMID: 34201415
- PMCID: PMC8269184
- DOI: 10.3390/ijms22136703
Do Changes in ACE-2 Expression Affect SARS-CoV-2 Virulence and Related Complications: A Closer Look into Membrane-Bound and Soluble Forms
Abstract
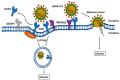
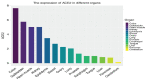
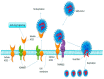
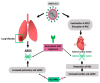
The SARS-CoV-2 virus utilizes angiotensin converting enzyme (ACE-2) for cell entry and infection. This enzyme has important functions in the renin-angiotensin aldosterone system to preserve cardiovascular function. In addition to the heart, it is expressed in many tissues including the lung, intestines, brain, and kidney, however, its functions in these organs are mostly unknown. ACE-2 has membrane-bound and soluble forms. Its expression levels are altered in disease states and by a variety of medications. Currently, it is not clear how altered ACE-2 levels influence ACE-2 virulence and relevant complications. In addition, membrane-bound and soluble forms are thought to have different effects. Most work on this topic in the literature is on the SARS-CoV virus that has a high genetic resemblance to SARS-Co-V-2 and also uses ACE-2 enzyme to enter the cell, but with much lower affinity. More recent studies on SARS-CoV-2 are mainly clinical studies aiming at relating the effect of medications that are thought to influence ACE-2 levels, with COVID-19 outcomes for patients under these medications. This review paper aims to summarize what is known about the relationship between ACE-2 levels and SARS-CoV/SARS-CoV-2 virulence under altered ACE-2 expression states.
Keywords: ACE inhibitors; ACE-2; ARDS; COVID-19; RAAS; SARS-CoV; angiotensin receptor blockers; shedding.
Conflict of interest statement
The author has no conflict of interest to declare with respect to this manuscript.
Figures



References
-
- Zhou L., Niu Z., Jiang X., Zhang Z., Zheng Y., Wang Z., Zhu Y., Gao L., Wang X., Sun Q. Systemic analysis of tissue cells potentially vulnerable to SARS-CoV-2 infection by the protein-proofed single-cell RNA profiling of ACE2, TMPRSS2 and Furin proteases. bioRxiv. 2020 doi: 10.1101/2020.04.06.028522. - DOI - PMC - PubMed
-
- Bos J.M., Hebl V.B., Oberg A.L., Sun Z., Herman D.S., Teekakirikul P., Seidman J.G., Seidman C.E., Dos Remedios C.G., Maleszewski J.J., et al. Marked Up-Regulation of ACE2 in Hearts of Patients With Obstructive Hypertrophic Cardiomyopathy: Implications for SARS-CoV-2-Mediated COVID-19. Mayo Clin. Proc. 2020;95:1354–1368. doi: 10.1016/j.mayocp.2020.04.028. - DOI - PMC - PubMed
-
- Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A., Diz D.I., Gallagher P.E. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

