Dual Mode of Action of Acetylcholine on Cytosolic Calcium Oscillations in Pancreatic Beta and Acinar Cells In Situ
- PMID: 34201461
- PMCID: PMC8305080
- DOI: 10.3390/cells10071580
Dual Mode of Action of Acetylcholine on Cytosolic Calcium Oscillations in Pancreatic Beta and Acinar Cells In Situ
Abstract
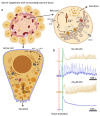
Cholinergic innervation in the pancreas controls both the release of digestive enzymes to support the intestinal digestion and absorption, as well as insulin release to promote nutrient use in the cells of the body. The effects of muscarinic receptor stimulation are described in detail for endocrine beta cells and exocrine acinar cells separately. Here we describe morphological and functional criteria to separate these two cell types in situ in tissue slices and simultaneously measure their response to ACh stimulation on cytosolic Ca2+ oscillations [Ca2+]c in stimulatory glucose conditions. Our results show that both cell types respond to glucose directly in the concentration range compatible with the glucose transporters they express. The physiological ACh concentration increases the frequency of glucose stimulated [Ca2+]c oscillations in both cell types and synchronizes [Ca2+]c oscillations in acinar cells. The supraphysiological ACh concentration further increases the oscillation frequency on the level of individual beta cells, inhibits the synchronization between these cells, and abolishes oscillatory activity in acinar cells. We discuss possible mechanisms leading to the observed phenomena.
Keywords: Ca2+ oscillations; acetylcholine; acinar cell; beta cell; pancreas tissue slices.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

