Assessment of Biotechnologically Important Filamentous Fungal Biomass by Fourier Transform Raman Spectroscopy
- PMID: 34201486
- PMCID: PMC8269384
- DOI: 10.3390/ijms22136710
Assessment of Biotechnologically Important Filamentous Fungal Biomass by Fourier Transform Raman Spectroscopy
Abstract
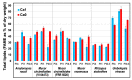
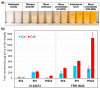
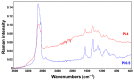
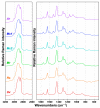
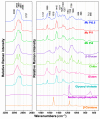
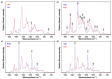
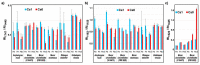
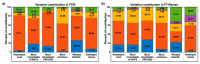
Oleaginous filamentous fungi can accumulate large amount of cellular lipids and biopolymers and pigments and potentially serve as a major source of biochemicals for food, feed, chemical, pharmaceutical, and transport industries. We assessed suitability of Fourier transform (FT) Raman spectroscopy for screening and process monitoring of filamentous fungi in biotechnology. Six Mucoromycota strains were cultivated in microbioreactors under six growth conditions (three phosphate concentrations in the presence and absence of calcium). FT-Raman and FT-infrared (FTIR) spectroscopic data was assessed in respect to reference analyses of lipids, phosphorus, and carotenoids by using principal component analysis (PCA), multiblock or consensus PCA, partial least square regression (PLSR), and analysis of spectral variation due to different design factors by an ANOVA model. All main chemical biomass constituents were detected by FT-Raman spectroscopy, including lipids, proteins, cell wall carbohydrates, and polyphosphates, and carotenoids. FT-Raman spectra clearly show the effect of growth conditions on fungal biomass. PLSR models with high coefficients of determination (0.83-0.94) and low error (approximately 8%) for quantitative determination of total lipids, phosphates, and carotenoids were established. FT-Raman spectroscopy showed great potential for chemical analysis of biomass of oleaginous filamentous fungi. The study demonstrates that FT-Raman and FTIR spectroscopies provide complementary information on main fungal biomass constituents.
Keywords: biodiesel; biopolymers; carotenoids; chitin; chitosan; fatty acids; fermentation; fungi; oleaginous microorganisms; pigments.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Meyer V., Andersen M.R., Brakhage A.A., Braus G.H., Caddick M.X., Cairns T.C., de Vries R.P., Haarmann T., Hansen K., Hertz-Fowler C., et al. Current challenges of research on filamentous fungi in relation to human welfare and a sustainable bio-economy: A white paper. Fungal Biol. Biotechnol. 2016;3:6. doi: 10.1186/s40694-016-0024-8. - DOI - PMC - PubMed
-
- Gupta V.K., Treichel H., Shapaval V., Oliveira L.A.d., Tuohy M.G. Microbial Functional Foods and Nutraceuticals. John Wiley & Sons; Hoboken, NJ, USA: 2017. pp. 1–309.
-
- Papanikolaou S., Galiotou-Panayotou M., Fakas S., Komaitis M., Aggelis G. Lipid production by oleaginous Mucorales cultivated on renewable carbon sources. Eur. J. Lipid Sci. Technol. 2007;109:1060–1070. doi: 10.1002/ejlt.200700169. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

