Morphological and Calcium Signaling Alterations of Neuroglial Cells in Cerebellar Cortical Dysplasia Induced by Carmustine
- PMID: 34201497
- PMCID: PMC8304447
- DOI: 10.3390/cells10071581
Morphological and Calcium Signaling Alterations of Neuroglial Cells in Cerebellar Cortical Dysplasia Induced by Carmustine
Abstract
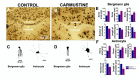
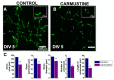
Cortical dysplasias are alterations in the organization of the layers of the brain cortex due to problems in neuronal migration during development. The neuronal component has been widely studied in experimental models of cortical dysplasias. In contrast, little is known about how glia are affected. In the cerebellum, Bergmann glia (BG) are essential for neuronal migration during development, and in adult they mediate the control of fine movements through glutamatergic transmission. The aim of this study was to characterize the morphology and intracellular calcium dynamics of BG and astrocytes from mouse cerebellum and their modifications in a model of cortical dysplasia induced by carmustine (BCNU). Carmustine-treated mice were affected in their motor coordination and balance. Cerebellar dysplasias and heterotopias were more frequently found in lobule X. Morphology of BG cells and astrocytes was affected, as were their spontaneous [Ca2+]i transients in slice preparation and in vitro.
Keywords: Bergmann glia; astrocytes; clarity; light-sheet fluorescence microscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
The effect of carmustine on Bergmann cells of the cerebellum.Neurosci Lett. 2015 May 19;595:18-24. doi: 10.1016/j.neulet.2015.03.068. Epub 2015 Apr 1. Neurosci Lett. 2015. PMID: 25841791
-
Expression of layer-specific markers in the adult neocortex of BCNU-Treated rat, a model of cortical dysplasia.Neuroscience. 2009 Mar 17;159(2):682-91. doi: 10.1016/j.neuroscience.2008.12.064. Epub 2009 Jan 10. Neuroscience. 2009. PMID: 19174181
-
Altered spatial distribution of PV-cortical cells and dysmorphic neurons in the somatosensory cortex of BCNU-treated rat model of cortical dysplasia.Epilepsia. 2008 May;49(5):872-87. doi: 10.1111/j.1528-1167.2007.01440.x. Epub 2007 Dec 10. Epilepsia. 2008. PMID: 18076647
-
Bergmann Glia, Long-Term Depression, and Autism Spectrum Disorder.Mol Neurobiol. 2017 Mar;54(2):1156-1166. doi: 10.1007/s12035-016-9719-3. Epub 2016 Jan 26. Mol Neurobiol. 2017. PMID: 26809583 Free PMC article. Review.
-
Origin, lineage and function of cerebellar glia.Prog Neurobiol. 2013 Oct;109:42-63. doi: 10.1016/j.pneurobio.2013.08.001. Epub 2013 Aug 25. Prog Neurobiol. 2013. PMID: 23981535 Review.
Cited by
-
Calcium Signaling in Astrocytes and Its Role in the Central Nervous System Injury.Mol Neurobiol. 2025 May 26. doi: 10.1007/s12035-025-05055-5. Online ahead of print. Mol Neurobiol. 2025. PMID: 40419752 Review.
-
Organization of the ventricular zone of the cerebellum.Front Cell Neurosci. 2022 Jul 25;16:955550. doi: 10.3389/fncel.2022.955550. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35959470 Free PMC article. Review.
-
The Function of Glial Cells in the Neuroinflammatory and Neuroimmunological Responses.Cells. 2022 Feb 14;11(4):659. doi: 10.3390/cells11040659. Cells. 2022. PMID: 35203307 Free PMC article.
References
-
- Moroni R.F., Inverardi F., Regondi M.C., Ferrucio P., Spreafico R., Frassoni C. Altered spatial distribution of PV-cortical cells and dysmorphic neurons in the somatosensory cortex of BCNU-treated rat model of cortical dysplasia. Epilepsy. 2008;49:882–887. doi: 10.1111/j.1528-1167.2007.01440.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

