NEUROD1 Is Required for the Early α and β Endocrine Differentiation in the Pancreas
- PMID: 34201511
- PMCID: PMC8268837
- DOI: 10.3390/ijms22136713
NEUROD1 Is Required for the Early α and β Endocrine Differentiation in the Pancreas
Abstract
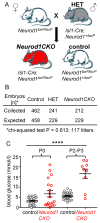
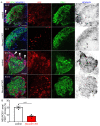
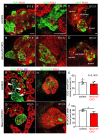
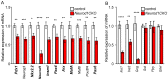
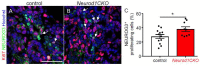
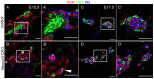
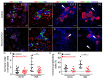
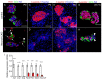
Diabetes is a metabolic disease that involves the death or dysfunction of the insulin-secreting β cells in the pancreas. Consequently, most diabetes research is aimed at understanding the molecular and cellular bases of pancreatic development, islet formation, β-cell survival, and insulin secretion. Complex interactions of signaling pathways and transcription factor networks regulate the specification, growth, and differentiation of cell types in the developing pancreas. Many of the same regulators continue to modulate gene expression and cell fate of the adult pancreas. The transcription factor NEUROD1 is essential for the maturation of β cells and the expansion of the pancreatic islet cell mass. Mutations of the Neurod1 gene cause diabetes in humans and mice. However, the different aspects of the requirement of NEUROD1 for pancreas development are not fully understood. In this study, we investigated the role of NEUROD1 during the primary and secondary transitions of mouse pancreas development. We determined that the elimination of Neurod1 impairs the expression of key transcription factors for α- and β-cell differentiation, β-cell proliferation, insulin production, and islets of Langerhans formation. These findings demonstrate that the Neurod1 deletion altered the properties of α and β endocrine cells, resulting in severe neonatal diabetes, and thus, NEUROD1 is required for proper activation of the transcriptional network and differentiation of functional α and β cells.
Keywords: NEUROD1; genetic mutation; mouse model; pancreatic development; transcriptional network.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Regulation of Neurod1 contributes to the lineage potential of Neurogenin3+ endocrine precursor cells in the pancreas.PLoS Genet. 2013;9(2):e1003278. doi: 10.1371/journal.pgen.1003278. Epub 2013 Feb 7. PLoS Genet. 2013. PMID: 23408910 Free PMC article.
-
Cooperative transcriptional regulation of the essential pancreatic islet gene NeuroD1 (beta2) by Nkx2.2 and neurogenin 3.J Biol Chem. 2009 Nov 6;284(45):31236-48. doi: 10.1074/jbc.M109.048694. Epub 2009 Sep 15. J Biol Chem. 2009. PMID: 19759004 Free PMC article.
-
Murine Perinatal β-Cell Proliferation and the Differentiation of Human Stem Cell-Derived Insulin-Expressing Cells Require NEUROD1.Diabetes. 2019 Dec;68(12):2259-2271. doi: 10.2337/db19-0117. Epub 2019 Sep 13. Diabetes. 2019. PMID: 31519700 Free PMC article.
-
Islet cell development.Adv Exp Med Biol. 2010;654:59-75. doi: 10.1007/978-90-481-3271-3_4. Adv Exp Med Biol. 2010. PMID: 20217494 Review.
-
Systematic single-cell analysis provides new insights into heterogeneity and plasticity of the pancreas.Mol Metab. 2017 Jul 20;6(9):974-990. doi: 10.1016/j.molmet.2017.06.021. eCollection 2017 Sep. Mol Metab. 2017. PMID: 28951822 Free PMC article. Review.
Cited by
-
Maturity-Onset Diabetes of the Young: Mutations, Physiological Consequences, and Treatment Options.J Pers Med. 2022 Oct 25;12(11):1762. doi: 10.3390/jpm12111762. J Pers Med. 2022. PMID: 36573710 Free PMC article. Review.
-
Induction of diabetes by Tacrolimus in a phenotypic model of obesity and metabolic syndrome.Front Endocrinol (Lausanne). 2024 Apr 29;15:1388361. doi: 10.3389/fendo.2024.1388361. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38745946 Free PMC article.
-
The expression order determines the pioneer functions of NGN3 and NEUROD1 in pancreatic endocrine differentiation.Sci Adv. 2025 Mar 28;11(13):eadt4770. doi: 10.1126/sciadv.adt4770. Epub 2025 Mar 26. Sci Adv. 2025. PMID: 40138419 Free PMC article.
-
Integrative single-cell characterization of a frugivorous and an insectivorous bat kidney and pancreas.Nat Commun. 2024 Jan 9;15(1):12. doi: 10.1038/s41467-023-44186-y. Nat Commun. 2024. PMID: 38195585 Free PMC article.
-
Identification of gene mutations associated with type 1 diabetes by next-generation sequencing in affected Palestinian families.Front Genet. 2024 Jan 11;14:1292073. doi: 10.3389/fgene.2023.1292073. eCollection 2023. Front Genet. 2024. PMID: 38274107 Free PMC article.
References
-
- Johansson K.A., Dursun U., Jordan N., Gu G., Beermann F., Gradwohl G., Grapin-Botton A. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev. Cell. 2007;12:457–465. doi: 10.1016/j.devcel.2007.02.010. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

