Analysis of the Role of FRMD5 in the Biology of Papillary Thyroid Carcinoma
- PMID: 34201607
- PMCID: PMC8268710
- DOI: 10.3390/ijms22136726
Analysis of the Role of FRMD5 in the Biology of Papillary Thyroid Carcinoma
Abstract
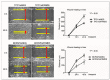
Background: Thyroid carcinoma (TC) is the most common endocrine system malignancy, and papillary thyroid carcinoma (PTC) accounts for >80% of all TC cases. Nevertheless, PTC pathogenesis is still not fully understood. The aim of the study was to elucidate the role of the FRMD5 protein in the regulation of biological pathways associated with the development of PTC. We imply that the presence of certain genetic aberrations (e.g., BRAF V600E mutation) is associated with the activity of FRMD5.
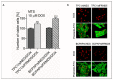
Methods: The studies were conducted on TPC1 and BCPAP (BRAF V600E) model PTC-derived cells. Transfection with siRNA was used to deplete the expression of FRMD5. The mRNA expression and protein yield were evaluated using RT-qPCR and Western blot techniques. Proliferation, migration, invasiveness, adhesion, spheroid formation, and survival tests were performed. RNA sequencing and phospho-kinase proteome profiling were used to assess signaling pathways associated with the FRMD5 expressional status.
Results: The obtained data indicate that the expression of FRMD5 is significantly enhanced in BRAF V600E tumor specimens and cells. It was observed that a drop in intracellular yield of FRMD5 results in significant alternations in the migration, invasiveness, adhesion, and spheroid formation potential of PTC-derived cells. Importantly, significant divergences in the effect of FRMD5 depletion in both BRAF-wt and BRAF-mutated PTC cells were observed. It was also found that knockdown of FRMD5 significantly alters the expression of multidrug resistant genes.
Conclusions: This is the first report highlighting the importance of the FRMD5 protein in the biology of PTCs. The results suggest that the FRMD5 protein can play an important role in controlling the metastatic potential and multidrug resistance of thyroid tumor cells.
Keywords: BRAF V600E; FRMD5; RNA sequencing; migration; papillary thyroid carcinoma.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures










References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

