Antibacterial Residue Excretion via Urine as an Indicator for Therapeutical Treatment Choice and Farm Waste Treatment
- PMID: 34201627
- PMCID: PMC8300810
- DOI: 10.3390/antibiotics10070762
Antibacterial Residue Excretion via Urine as an Indicator for Therapeutical Treatment Choice and Farm Waste Treatment
Abstract
Many of the infectious diseases that affect livestock have bacteria as etiological agents. Thus, therapy is based on antimicrobials that leave the animal's tissues mainly via urine, reaching the environment through slurry and waste water. Once there, antimicrobial residues may lead to antibacterial resistance as well as toxicity for plants, animals, or humans. Hence, the objective was to describe the rate of antimicrobial excretion in urine in order to select the most appropriate molecule while reducing harmful effects. Thus, 62 pigs were treated with sulfamethoxypyridazine, oxytetracycline, and enrofloxacin. Urine was collected through the withdrawal period and analysed via LC-MS/MS. Oxytetracycline had the slowest rate of degradation (a half-life time of 4.18 days) and the most extended elimination period in urine (over 2 months), followed by enrofloxacin (a half-life time of 1.48 days, total urine elimination in ca. 3 weeks) and sulfamethoxypyridazine (a half-life time of 0.49 days, total urine elimination in ca. 1 week). Bacterial sensitivity and recommendations for responsible use are limiting when selecting the treatment. Nevertheless, with similar effectiveness, sulfamethoxypyridazine would be the choice, as waste treatment would only need to be implemented for 1 week after treatment. Thus, more in-depth knowledge regarding antibacterial elimination would improve resource management, while protecting animals and consumers' health.
Keywords: LC–MS/MS; antibiotic; excretion; quinolone; sulfonamide; urine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
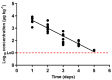



 ), oxytetracycline (
), oxytetracycline ( ), and enrofloxacin (
), and enrofloxacin ( ) in urine after animal treatment within the withdrawal period. The LoD dotted line represents the detection limit of the analytical technique for sulfamethoxypyridazine, oxytetracycline, and enrofloxacin.
) in urine after animal treatment within the withdrawal period. The LoD dotted line represents the detection limit of the analytical technique for sulfamethoxypyridazine, oxytetracycline, and enrofloxacin.Similar articles
-
Is Blood a Good Indicator for Detecting Antimicrobials in Meat? Evidence for the Development of In Vivo Surveillance Methods.Antibiotics (Basel). 2020 Apr 12;9(4):175. doi: 10.3390/antibiotics9040175. Antibiotics (Basel). 2020. PMID: 32290542 Free PMC article.
-
Epidemiological assessment of antibiotic residues in dairy farm milk and farm waste and water in northern India.Environ Sci Pollut Res Int. 2021 Jun;28(23):29455-29466. doi: 10.1007/s11356-020-12057-4. Epub 2021 Feb 9. Environ Sci Pollut Res Int. 2021. PMID: 33559823
-
Effect of Tetracycline Dose and Treatment Mode on Selection of Resistant Coliform Bacteria in Nursery Pigs.Appl Environ Microbiol. 2017 May 31;83(12):e00538-17. doi: 10.1128/AEM.00538-17. Print 2017 Jun 15. Appl Environ Microbiol. 2017. PMID: 28389548 Free PMC article. Clinical Trial.
-
Mitoxantrone: a review of its use in multiple sclerosis.CNS Drugs. 2004;18(6):379-96. doi: 10.2165/00023210-200418060-00010. CNS Drugs. 2004. PMID: 15089110 Review.
-
Antimicrobial resistance of mastitis pathogens.Vet Clin North Am Food Anim Pract. 2012 Jul;28(2):165-85. doi: 10.1016/j.cvfa.2012.03.005. Epub 2012 Apr 28. Vet Clin North Am Food Anim Pract. 2012. PMID: 22664201 Review.
Cited by
-
A novel portable Raman scattering platform for antibiotic screening in pig urine.Vet World. 2023 Jan;16(1):204-214. doi: 10.14202/vetworld.2023.204-214. Epub 2023 Jan 29. Vet World. 2023. PMID: 36855369 Free PMC article.
-
Efficient removal of ciprofloxacin from aqueous solution using Zn-C battery derived graphene oxide enhanced by hydrogen bonding, electrostatic and π-π interaction.Heliyon. 2024 Jun 21;10(12):e33317. doi: 10.1016/j.heliyon.2024.e33317. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 39022076 Free PMC article.
-
Antibiotic resistance and mitigation using One Health lens in aquaculture of Northern Nigeria.Onderstepoort J Vet Res. 2024 Oct 16;91(2):e1-e11. doi: 10.4102/ojvr.v91i2.2165. Onderstepoort J Vet Res. 2024. PMID: 39494642 Free PMC article.
References
-
- O’Neill J. The Review on Antimicrobial Resistance. Wellcome Trust, HM Government; London, UK: 2015. [(accessed on 19 November 2020)]. Antimicrobials in agriculture and the environment: Reducing unnecessary use and waste; pp. 1–44. Available online: https://amr-review.org/sites/default/files/Antimicrobials%20in%20agricul....
-
- Tang K.L., Caffrey N.P., Nobrega D.B., Cork S.C., Ronksley P.E., Barkema H.W., Polachek A.J., Ganshron H., Sharma N., Kellner J.D., et al. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: A systematic review and meta-analysis. Lancet Planet Health. 2017;1:316–327. doi: 10.1016/S2542-5196(17)30141-9. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

