The Erns Carboxyterminus: Much More Than a Membrane Anchor
- PMID: 34201636
- PMCID: PMC8310223
- DOI: 10.3390/v13071203
The Erns Carboxyterminus: Much More Than a Membrane Anchor
Abstract
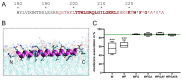
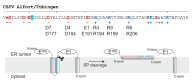
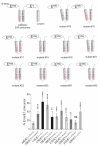
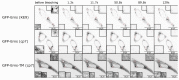
Pestiviruses express the unique essential envelope protein Erns, which exhibits RNase activity, is attached to membranes by a long amphipathic helix, and is partially secreted from infected cells. The RNase activity of Erns is directly connected with pestivirus virulence. Formation of homodimers and secretion of the protein are hypothesized to be important for its role as a virulence factor, which impairs the host's innate immune response to pestivirus infection. The unusual membrane anchor of Erns raises questions with regard to proteolytic processing of the viral polyprotein at the Erns carboxy-terminus. Moreover, the membrane anchor is crucial for establishing the critical equilibrium between retention and secretion and ensures intracellular accumulation of the protein at the site of virus budding so that it is available to serve both as structural component of the virion and factor controlling host immune reactions. In the present manuscript, we summarize published as well as new data on the molecular features of Erns including aspects of its interplay with the other two envelope proteins with a special focus on the biochemistry of the Erns membrane anchor.
Keywords: ER retention; RNA virus polyprotein processing; amphipathic helix; charge zipper; extracellular vesicles; membrane anchor; pestivirus; secreted T2 RNase; signal peptidase; transport of virus particles; virus assembly; virus budding.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures







References
-
- Lindenbach B.D., Thiel H.J., Rice C.M. Flaviviridae: The Viruses and Their Replication. In: Knipe D.M., Howley P.M., editors. Fields Virology. Volume 5. Lippincott—Raven Publishers; Philadelphia, PA, USA: 2007. pp. 1101–1152.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

