From Messengers to Receptors in Psoriasis: The Role of IL-17RA in Disease and Treatment
- PMID: 34201664
- PMCID: PMC8268646
- DOI: 10.3390/ijms22136740
From Messengers to Receptors in Psoriasis: The Role of IL-17RA in Disease and Treatment
Erratum in
-
Correction: Vidal et al. From Messengers to Receptors in Psoriasis: The Role of IL-17RA in Disease and Treatment. Int. J. Mol. Sci. 2021, 22, 6740.Int J Mol Sci. 2022 Feb 28;23(5):2649. doi: 10.3390/ijms23052649. Int J Mol Sci. 2022. PMID: 35270045 Free PMC article.
Abstract
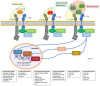
The paradigm of psoriasis as a Th17-driven disease has evolved in the last years towards a much deeper knowledge of the complex pathways, mechanisms, cells, and messengers involved, highlighting the crucial role played by the IL-17 family of cytokines. All IL-17 isoforms signal through IL-17R. Five subunits of IL-17R have been described to date, which couple to form a homo- or hetero-receptor complex. Characteristically, IL-17RA is a common subunit in all hetero-receptors. IL-17RA has unique structural-containing a SEFIR/TILL domain-and functional-requiring ACT-1 for signaling-properties, enabling Th17 cells to act as a bridge between innate and adaptive immune cells. In psoriasis, IL-17RA plays a key role in pathogenesis based on: (a) IL-17A, IL-17F, and other IL-17 isoforms are involved in disease development; and (b) IL-17RA is essential for signaling of all IL-17 cytokines but IL-17D, whose receptor has not been identified to date. This article reviews current evidence on the biology and role of the IL-17 family of cytokines and receptors, with focus on IL-17RA, in psoriasis and some related comorbidities, and puts them in context with current and upcoming treatments.
Keywords: IL-17; IL-17R; Th17; bimekizumab; brodalumab; ixekizumab; monoclonal antibodies; psoriasis; secukinumab.
Conflict of interest statement
Silvia Vidal: Her institution has received grants or contracts from Novartis, BMS, Roche, Almirall, and Precigen, and she has received payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events from Sanofi, Eusa-pharma, Novartis, Roche, and Precigen. José-Manuel Carrascosa-Carrillo: His institution has received grants, has given research support, or participation in clinical trials from Abbvie, Almirall, Amgen, Boehringer Ingelheim, Celgene, Janssen, Leo-Pharma, Lilly, Novartis, Pfizer, Sanofi, and Sandoz. He has received honoraria or consultation fees from Abbvie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Fresenius-Kabi, Janssen, Leo-Pharma, Lilly, Mylan, Novartis, Pfizer, Sandoz, Samsung-Bioepis, Sanofi, and UCB. He has participated in a company sponsored speaker’s bureau: Celgene, Janssen, Lilly, Novartis, Pfizer, Almirall, and UCB. Álvaro González-Cantero: His institution has received grants or contracts from: Leo-Pharma, Almirall, and Celgene. He has received payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events from: Abbie, Janssen, Novartis, Almirall, Celgene, UCB, L´Oreal, MSD, and Leo Pharma. He has been given support for attending meetings and/or travel from: Abbvie, Janssen, Novartis, Almirall, Celgene, UCB, and Leo Pharma. He has participated on a Data Safety Monitoring Board or Advisory Board for: Abbie, Janssen, Novartis, Almirall, Celgene, UCB, L´Oreal, MSD, and Leo Pharma. Lluís Puig: His institution has received grants/research supports or participation in clinical trials from: Abbvie, Almirall, Amgen, Boehringer Ingelheim, Celgene, Janssen, Leo-Pharma, Lilly, Novartis, Pfizer, Regeneron, Roche, Sanofi, and UCB. He has received honoraria or consultation fees from: Abbvie, Almirall, Amgen, Baxalta, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Fresenius-Kabi, Janssen, JS BIOCAD, Leo-Pharma, Lilly, Mylan, Novartis, Pfizer, Regeneron, Roche, Sandoz, Samsung-Bioepis, Sanofi, and UCB. He has participated in a company sponsored speaker’s bureau: Celgene, Janssen, Lilly, Novartis, and Pfizer. José-Carlos Ruiz-Carrascosa: The author declares no conflict of interest. Antonio-Manuel Velasco-Pastor has received fees for advice and speeches from Allmirall, Leo Pharma, Novartis, Janssen, Lilly, Abbvie, Gebro, Pfizer, Bristol, and Amgen.
Figures
References
-
- Bjerke J.R. In Situ Characterization and Counting of Mononuclear Cells in Lesions of Different Clinical Forms of Psoriasis. Acta Derm Venereol. 1982;62:93–100. - PubMed

