Metabolic Dynamics in Short- and Long-Term Microgravity in Human Primary Macrophages
- PMID: 34201720
- PMCID: PMC8269311
- DOI: 10.3390/ijms22136752
Metabolic Dynamics in Short- and Long-Term Microgravity in Human Primary Macrophages
Abstract
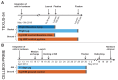
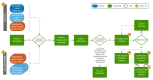
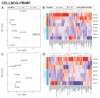
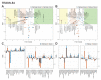
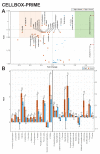
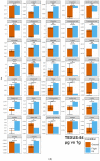
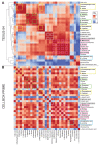
Microgravity acts on cellular systems on several levels. Cells of the immune system especially react rapidly to changes in gravity. In this study, we performed a correlative metabolomics analysis on short-term and long-term microgravity effects on primary human macrophages. We could detect an increased amino acid concentration after five minutes of altered gravity, that was inverted after 11 days of microgravity. The amino acids that reacted the most to changes in gravity were tightly clustered. The observed effects indicated protein degradation processes in microgravity. Further, glucogenic and ketogenic amino acids were further degraded to Glucose and Ketoleucine. The latter is robustly accumulated in short-term and long-term microgravity but not in hypergravity. We detected highly dynamic and also robust adaptative metabolic changes in altered gravity. Metabolomic studies could contribute significantly to the understanding of gravity-induced integrative effects in human cells.
Keywords: immune cells; metabolomics; microgravity; sounding rocket; spaceflight.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Parabolic, Flight-Induced, Acute Hypergravity and Microgravity Effects on the Beating Rate of Human Cardiomyocytes.Cells. 2019 Apr 14;8(4):352. doi: 10.3390/cells8040352. Cells. 2019. PMID: 31013958 Free PMC article.
-
Modulation of Differentiation Processes in Murine Embryonic Stem Cells Exposed to Parabolic Flight-Induced Acute Hypergravity and Microgravity.Stem Cells Dev. 2018 Jun 15;27(12):838-847. doi: 10.1089/scd.2017.0294. Epub 2018 Apr 9. Stem Cells Dev. 2018. PMID: 29630478 Free PMC article.
-
Cytoskeletal stability and metabolic alterations in primary human macrophages in long-term microgravity.PLoS One. 2017 Apr 18;12(4):e0175599. doi: 10.1371/journal.pone.0175599. eCollection 2017. PLoS One. 2017. PMID: 28419128 Free PMC article.
-
The brain in micro- and hypergravity: the effects of changing gravity on the brain electrocortical activity.Eur J Sport Sci. 2014;14(8):813-22. doi: 10.1080/17461391.2014.908959. Epub 2014 Apr 15. Eur J Sport Sci. 2014. PMID: 24734884 Review.
-
Reproductive hazards of space travel in women and men.Nat Rev Endocrinol. 2019 Dec;15(12):713-730. doi: 10.1038/s41574-019-0267-6. Epub 2019 Oct 14. Nat Rev Endocrinol. 2019. PMID: 31611649 Free PMC article. Review.
Cited by
-
Omics Studies of Specialized Cells and Stem Cells under Microgravity Conditions.Int J Mol Sci. 2024 Sep 17;25(18):10014. doi: 10.3390/ijms251810014. Int J Mol Sci. 2024. PMID: 39337501 Free PMC article. Review.
-
Synergistic interplay between radiation and microgravity in spaceflight-related immunological health risks.Immun Ageing. 2024 Jul 20;21(1):50. doi: 10.1186/s12979-024-00449-w. Immun Ageing. 2024. PMID: 39033285 Free PMC article. Review.
-
Taking the 3Rs to a higher level: replacement and reduction of animal testing in life sciences in space research.Biotechnol Adv. 2025 Jul-Aug;81:108574. doi: 10.1016/j.biotechadv.2025.108574. Epub 2025 Apr 1. Biotechnol Adv. 2025. PMID: 40180136 Review.
-
MRTF may be the missing link in a multiscale mechanobiology approach toward macrophage dysfunction in space.Front Cell Dev Biol. 2022 Sep 12;10:997365. doi: 10.3389/fcell.2022.997365. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36172272 Free PMC article.
-
Impacts of microgravity on amino acid metabolism during spaceflight.Exp Biol Med (Maywood). 2023 May;248(5):380-393. doi: 10.1177/15353702221139189. Epub 2023 Feb 12. Exp Biol Med (Maywood). 2023. PMID: 36775855 Free PMC article. Review.
References
-
- Kimzey S.L. Biomedical Results from Skylab. NASA; Washington, DC, USA: 1977. Hematology and immunology studies.
-
- NASA Manned Spacecraft Center . Gemini Summary Conference. NASA; Houston, TX, USA: 1967.
-
- Konstantinova I.V., Antropova Y.N., Legenkov V.I., Zazhirey V.D. Study of reactivity of blood lymphoid cells in crew members of the Soyuz-6, Soyuz-7 and Soyuz-8 spaceships before and after flight(blatogenesis delay of lymphocytes) Space Biol. Med. 1973;7:48–55.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

