Use of Ceftaroline in Hospitalized Patients with and without COVID-19: A Descriptive Cross-Sectional Study
- PMID: 34201722
- PMCID: PMC8300614
- DOI: 10.3390/antibiotics10070763
Use of Ceftaroline in Hospitalized Patients with and without COVID-19: A Descriptive Cross-Sectional Study
Abstract
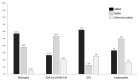
A single-center cross-sectional study was conducted to describe the use of ceftaroline in a large teaching hospital in Northern Italy, during a period also including the first months of the coronavirus disease 2019 (COVID-19) pandemic. The primary objective was to describe the use of ceftaroline in terms of indications and characteristics of patients. A secondary objective was to describe the rate of favorable clinical response in patients with bloodstream infections (BSI) due to methicillin-resistant Staphylococcus aureus (MRSA-BSI) receiving ceftaroline. Overall, 200 patients were included in the study. Most of them had COVID-19 (83%, 165/200) and were hospitalized in medical wards (78%, 155/200). Included patients with COVID-19 pneumonia were given empirical ceftaroline in the suspicion of bacterial co-infection or superinfection. Among patients with MRSA-BSI, ceftaroline was used as a first-line therapy and salvage therapy in 25% (3/12) and 75% (9/12) of cases, respectively, and as a monotherapy or in combination with daptomycin in 58% (7/12) and 42% (5/12) of patients, respectively. A favorable response was registered in 67% (8/12) of patients. Improving etiological diagnosis of bacterial infections is essential to optimize the use of ceftaroline in COVID-19 patients. The use of ceftaroline for MRSA-BSI, either as a monotherapy or in combination with other anti-MRSA agents, showed promising rates of favorable response.
Keywords: COVID-19; MRSA; Staphylococcus aureus; ceftaroline.
Conflict of interest statement
Outside the submitted work, D.R.G. reports honoraria from Stepstone Pharma GmbH and unconditional grants from MSD Italia and Correvio Italia. Outside the submitted work, M.B. (Matteo Bassetti) has received funding for scientific advisory boards, travel and speaker honoraria from Angelini, Astellas, AstraZeneca, Basilea, Bayer, BioMérieux, Cidara, Correvio, Cubist, Menarini, Molteni, MSD, Nabriva, Paratek, Pfizer, Roche, Shionogi, Tetraphase, Thermo Fisher, and The Medicine Company. All the other authors declare no conflicts of interest.
Figures
References
-
- European Medicines Agency Zinforo 600 mg Powder for Concentrate for Solution for Infusion: Summary of Product Characteristics. [(accessed on 1 May 2021)]; Available online: https://www.ema.europa.eu/en/documents/product-information/zinforo-epar-....
-
- Bassetti M., Russo A., Cilloniz C., Giacobbe D.R., Vena A., Amaro R., Graziano E., Soriano A., Torres A. Ceftaroline for severe community-acquired pneumonia: A real-world two-centre experience in Italy and Spain. Int. J. Antimicrob. Agents. 2020;55:105921. doi: 10.1016/j.ijantimicag.2020.105921. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

