Feasibility of Secondary Follicle Isolation, Culture and Achievement of In-Vitro Oocyte Maturation from Superovulated Ovaries: An Experimental Proof-of-Concept Study Using Mice
- PMID: 34201725
- PMCID: PMC8268951
- DOI: 10.3390/jcm10132757
Feasibility of Secondary Follicle Isolation, Culture and Achievement of In-Vitro Oocyte Maturation from Superovulated Ovaries: An Experimental Proof-of-Concept Study Using Mice
Abstract
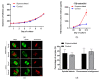
Fertility preservation through ovarian stimulation, aiming at cryopreserving mature oocytes or embryos, is sometimes unsuccessful. This clinical situation deserves novel approaches to overcome infertility following cancer treatment in patients facing highly gonadotoxic treatment. In this controlled experimental study, we investigated the feasibility of in-vitro culturing secondary follicles isolated from superovulated ovaries of mice recently treated with gonadotropins. The follicle yields of superovulated ovaries were 45.9% less than in unstimulated controls. Follicles from superovulated ovaries showed faster growth pace during the initial 7 days of culture and secreted more 17β-estradiol by the end of culture vs controls. Parameters reflecting the outcome of follicular development and oocyte maturation competence in vitro were similar between superovulated and control groups, with a similar follicle size at the end of culture and around 70% survival. Nearly half of cultured follicles met the criteria for in-vitro maturation in both groups and approximately 60% of those achieved a mature MII oocyte, similarly in both groups. Over 60% of obtained MII oocytes displayed normal-looking spindle and chromosome configurations, without significant differences between the groups. Using a validated follicle culture system, we demonstrated the feasibility of secondary follicle isolation, in-vitro culture and oocyte maturation with normal spindle and chromosome configurations obtained from superovulated mice ovaries.
Keywords: female fertility preservation; follicle isolation; in-vitro maturation; ovarian tissue; secondary follicle culture; superovulation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- World Health Organization. [(accessed on 21 January 2021)]; Available online: https://www.who.int/health-topics/cancer#tab=tab_1.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

