The Effect of DREADD Activation of Leptin Receptor Positive Neurons in the Nucleus of the Solitary Tract on Sleep Disordered Breathing
- PMID: 34201760
- PMCID: PMC8269100
- DOI: 10.3390/ijms22136742
The Effect of DREADD Activation of Leptin Receptor Positive Neurons in the Nucleus of the Solitary Tract on Sleep Disordered Breathing
Abstract
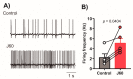
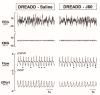
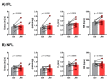
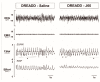
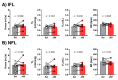
Obstructive sleep apnea (OSA) is recurrent obstruction of the upper airway due to the loss of upper airway muscle tone during sleep. OSA is highly prevalent, especially in obesity. There is no pharmacotherapy for OSA. Previous studies have demonstrated the role of leptin, an adipose-tissue-produced hormone, as a potent respiratory stimulant. Leptin signaling via a long functional isoform of leptin receptor, LEPRb, in the nucleus of the solitary tract (NTS), has been implicated in control of breathing. We hypothesized that leptin acts on LEPRb positive neurons in the NTS to increase ventilation and maintain upper airway patency during sleep in obese mice. We expressed designer receptors exclusively activated by designer drugs (DREADD) selectively in the LEPRb positive neurons of the NTS of Leprb-Cre-GFP mice with diet-induced obesity (DIO) and examined the effect of DREADD ligand, J60, on tongue muscle activity and breathing during sleep. J60 was a potent activator of LEPRb positive NTS neurons, but did not stimulate breathing or upper airway muscles during NREM and REM sleep. We conclude that, in DIO mice, the stimulating effects of leptin on breathing during sleep are independent of LEPRb signaling in the NTS.
Keywords: chemogenetics; obstructive sleep apnea; upper airway dysfunction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Heinzer R., Vat S., Marques-Vidal P., Marti-Soler H., Andries D., Tobback N., Mooser V., Preisig M., Malhotra A., Waeber G., et al. Prevalence of Sleep-Disordered Breathing in the General Population: The HypnoLaus Study. Lancet Respir. Med. 2015;3:310–318. doi: 10.1016/S2213-2600(15)00043-0. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

