Hybrid Methacrylated Gelatin and Hyaluronic Acid Hydrogel Scaffolds. Preparation and Systematic Characterization for Prospective Tissue Engineering Applications
- PMID: 34201769
- PMCID: PMC8268476
- DOI: 10.3390/ijms22136758
Hybrid Methacrylated Gelatin and Hyaluronic Acid Hydrogel Scaffolds. Preparation and Systematic Characterization for Prospective Tissue Engineering Applications
Abstract
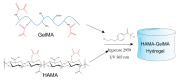
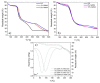
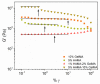
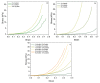
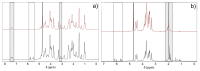
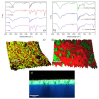
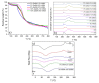
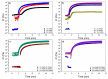
Hyaluronic acid (HA) and gelatin (Gel) are major components of the extracellular matrix of different tissues, and thus are largely appealing for the construction of hybrid hydrogels to combine the favorable characteristics of each biopolymer, such as the gel adhesiveness of Gel and the better mechanical strength of HA, respectively. However, despite previous studies conducted so far, the relationship between composition and scaffold structure and physico-chemical properties has not been completely and systematically established. In this work, pure and hybrid hydrogels of methacroyl-modified HA (HAMA) and Gel (GelMA) were prepared by UV photopolymerization and an extensive characterization was done to elucidate such correlations. Methacrylation degrees of ca. 40% and 11% for GelMA and HAMA, respectively, were obtained, which allows to improve the hydrogels' mechanical properties. Hybrid GelMA/HAMA hydrogels were stiffer, with elastic modulus up to ca. 30 kPa, and porous (up to 91%) compared with pure GelMA ones at similar GelMA concentrations thanks to the interaction between HAMA and GelMA chains in the polymeric matrix. The progressive presence of HAMA gave rise to scaffolds with more disorganized, stiffer, and less porous structures owing to the net increase of mass in the hydrogel compositions. HAMA also made hybrid hydrogels more swellable and resistant to collagenase biodegradation. Hence, the suitable choice of polymeric composition allows to regulate the hydrogels´ physical properties to look for the most optimal characteristics required for the intended tissue engineering application.
Keywords: gelatin; hyaluronic acid; hybrid scaffolds; hydrogel; porosity; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
- PID2019-109517RB-I00,/Agencia Estatal de Investigación
- MAT2016-8266R/Agencia Estatal de Investigación
- ED431C 2018/26/Consellería de Cultura, Educación e Ordenación Universitaria, Xunta de Galicia
- ED431C 2020/17/Consellería de Cultura, Educación e Ordenación Universitaria, Xunta de Galicia
- ISPP-144/International Scientific Partnership Program ISSP at King Saud University
LinkOut - more resources
Full Text Sources

