Investigating Immune Responses to the scAAV9- HEXM Gene Therapy Treatment in Tay-Sachs Disease and Sandhoff Disease Mouse Models
- PMID: 34201771
- PMCID: PMC8268035
- DOI: 10.3390/ijms22136751
Investigating Immune Responses to the scAAV9- HEXM Gene Therapy Treatment in Tay-Sachs Disease and Sandhoff Disease Mouse Models
Abstract
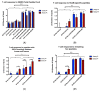
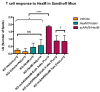
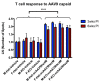
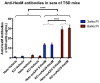
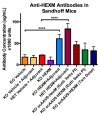
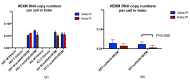
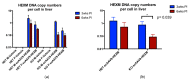
GM2 gangliosidosis disorders are a group of neurodegenerative diseases that result from a functional deficiency of the enzyme β-hexosaminidase A (HexA). HexA consists of an α- and β-subunit; a deficiency in either subunit results in Tay-Sachs Disease (TSD) or Sandhoff Disease (SD), respectively. Viral vector gene transfer is viewed as a potential method of treating these diseases. A recently constructed isoenzyme to HexA, called HexM, has the ability to effectively catabolize GM2 gangliosides in vivo. Previous gene transfer studies have revealed that the scAAV9-HEXM treatment can improve survival in the murine SD model. However, it is speculated that this treatment could elicit an immune response to the carrier capsid and "non-self"-expressed transgene. This study was designed to assess the immunocompetence of TSD and SD mice, and test the immune response to the scAAV9-HEXM gene transfer. HexM vector-treated mice developed a significant anti-HexM T cell response and antibody response. This study confirms that TSD and SD mouse models are immunocompetent, and that gene transfer expression can create an immune response in these mice. These mouse models could be utilized for investigating methods of mitigating immune responses to gene transfer-expressed "non-self" proteins, and potentially improve treatment efficacy.
Keywords: GM2; HexM; Sandhoff; Tay-Sachs; capsid; gangliosidosis; immunocompetence; murine; scAAV9-HEXM; transgene.
Conflict of interest statement
The authors declare the following conflict of interest: W.F.K. and J.G.K. are officials of New Hope Research Foundation, a 501(c)(3) non-profit operating foundation and the funding agency; J.G.K. is an inventor of the UsP promoter. The IP has been donated and assigned royalty-free to New Hope Research Foundation; S.J.G. received patent royalties for intellectual property (IP) licensed to Asklepios Biophama, however this IP was not used in this study; J.S.W. also has an unrelated IP, which has been licensed to Taysha Gene Therapies.
Figures
Similar articles
-
Novel Vector Design and Hexosaminidase Variant Enabling Self-Complementary Adeno-Associated Virus for the Treatment of Tay-Sachs Disease.Hum Gene Ther. 2016 Jul;27(7):509-21. doi: 10.1089/hum.2016.013. Hum Gene Ther. 2016. PMID: 27197548 Free PMC article.
-
Systemic Gene Transfer of a Hexosaminidase Variant Using an scAAV9.47 Vector Corrects GM2 Gangliosidosis in Sandhoff Mice.Hum Gene Ther. 2016 Jul;27(7):497-508. doi: 10.1089/hum.2016.015. Hum Gene Ther. 2016. PMID: 27199088
-
Pronounced Therapeutic Benefit of a Single Bidirectional AAV Vector Administered Systemically in Sandhoff Mice.Mol Ther. 2020 Oct 7;28(10):2150-2160. doi: 10.1016/j.ymthe.2020.06.021. Epub 2020 Jun 19. Mol Ther. 2020. PMID: 32592687 Free PMC article.
-
[Molecular pathogenesis and therapeutic approach of GM2 gangliosidosis].Yakugaku Zasshi. 2013;133(2):269-74. doi: 10.1248/yakushi.12-00199. Yakugaku Zasshi. 2013. PMID: 23370522 Review. Japanese.
-
[Recent advances in molecular genetics of GM2 gangliosidosis].Nihon Rinsho. 1995 Dec;53(12):2988-93. Nihon Rinsho. 1995. PMID: 8577047 Review. Japanese.
Cited by
-
A father's crusade in rare disease drug development: a case study of Elpida therapeutics and Melpida.Orphanet J Rare Dis. 2025 Jul 16;20(1):363. doi: 10.1186/s13023-025-03892-0. Orphanet J Rare Dis. 2025. PMID: 40665386 Free PMC article.
-
Increasing β-hexosaminidase A activity using genetically modified mesenchymal stem cells.Neural Regen Res. 2024 Jan;19(1):212-219. doi: 10.4103/1673-5374.375328. Neural Regen Res. 2024. PMID: 37488869 Free PMC article.
-
Intrathecal delivery of a bicistronic AAV9 vector expressing β-hexosaminidase A corrects Sandhoff disease in a murine model: A dosage study.Mol Ther Methods Clin Dev. 2023 Dec 5;32(1):101168. doi: 10.1016/j.omtm.2023.101168. eCollection 2024 Mar 14. Mol Ther Methods Clin Dev. 2023. PMID: 38205442 Free PMC article.
-
Efficacy of Adeno-Associated Virus Serotype 9-Mediated Gene Therapy for AB-Variant GM2 Gangliosidosis.Int J Mol Sci. 2023 Sep 27;24(19):14611. doi: 10.3390/ijms241914611. Int J Mol Sci. 2023. PMID: 37834060 Free PMC article.
-
Patients' view on gene therapy development for lysosomal storage disorders: a qualitative study.Orphanet J Rare Dis. 2022 Oct 21;17(1):383. doi: 10.1186/s13023-022-02543-y. Orphanet J Rare Dis. 2022. PMID: 36271424 Free PMC article.
References
-
- Myrianthopoulos N.C. Some Epidemiologic and Genetic Aspects of Tay Sachs Disease. Academic Press; New York, NY, USA: 1962.
-
- Gravel R.A., Clarke J.T.R., Kaback M.M., Mahuran D., Sandhoff K., Suzuki K. Chapter 92: The GM2 Gangliosidoses. In: Valle D., editor. Metabolic and Molecular Basis of Inherited Disease. 7th ed. McGraw-Hill Professional; New York, NY, USA: 1995. pp. 2839–2869.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

