Dexamethasone for Severe COVID-19: How Does It Work at Cellular and Molecular Levels?
- PMID: 34201797
- PMCID: PMC8269070
- DOI: 10.3390/ijms22136764
Dexamethasone for Severe COVID-19: How Does It Work at Cellular and Molecular Levels?
Abstract
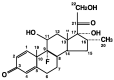
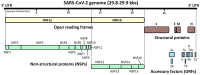
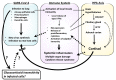
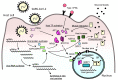
The coronavirus disease 2019 (COVID-19) caused by infection of the severe respiratory syndrome coronavirus-2 (SARS-CoV-2) significantly impacted human society. Recently, the synthetic pure glucocorticoid dexamethasone was identified as an effective compound for treatment of severe COVID-19. However, glucocorticoids are generally harmful for infectious diseases, such as bacterial sepsis and severe influenza pneumonia, which can develop respiratory failure and systemic inflammation similar to COVID-19. This apparent inconsistency suggests the presence of pathologic mechanism(s) unique to COVID-19 that renders this steroid effective. We review plausible mechanisms and advance the hypothesis that SARS-CoV-2 infection is accompanied by infected cell-specific glucocorticoid insensitivity as reported for some other viruses. This alteration in local glucocorticoid actions interferes with undesired glucocorticoid to facilitate viral replication but does not affect desired anti-inflammatory properties in non-infected organs/tissues. We postulate that the virus coincidentally causes glucocorticoid insensitivity in the process of modulating host cell activities for promoting its replication in infected cells. We explore this tenet focusing on SARS-CoV-2-encoding proteins and potential molecular mechanisms supporting this hypothetical glucocorticoid insensitivity unique to COVID-19 but not characteristic of other life-threatening viral diseases, probably due to a difference in specific virally-encoded molecules and host cell activities modulated by them.
Keywords: glucocorticoid receptor (GR); glucocorticoids; inflammation; innate immunity; severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2); type I interferons (IFNs).
Conflict of interest statement
The authors declare no conflict of interest. J.H.S. serves on the ASRM COVID-19 task force.
Figures
Similar articles
-
Antiviral Activity of Type I, II, and III Interferons Counterbalances ACE2 Inducibility and Restricts SARS-CoV-2.mBio. 2020 Sep 10;11(5):e01928-20. doi: 10.1128/mBio.01928-20. mBio. 2020. PMID: 32913009 Free PMC article.
-
Beneficial effect of combinational methylprednisolone and remdesivir in hamster model of SARS-CoV-2 infection.Emerg Microbes Infect. 2021 Dec;10(1):291-304. doi: 10.1080/22221751.2021.1885998. Emerg Microbes Infect. 2021. PMID: 33538646 Free PMC article.
-
Long Noncoding RNAs as Emerging Regulators of COVID-19.Front Immunol. 2021 Aug 2;12:700184. doi: 10.3389/fimmu.2021.700184. eCollection 2021. Front Immunol. 2021. PMID: 34408749 Free PMC article. Review.
-
Nonsteroidal anti-inflammatory drugs and glucocorticoids in COVID-19.Adv Biol Regul. 2021 Aug;81:100818. doi: 10.1016/j.jbior.2021.100818. Epub 2021 Jul 15. Adv Biol Regul. 2021. PMID: 34303107 Free PMC article. Review.
-
Immune-viral dynamics modeling for SARS-CoV-2 drug development.Clin Transl Sci. 2021 Nov;14(6):2348-2359. doi: 10.1111/cts.13099. Epub 2021 Jul 8. Clin Transl Sci. 2021. PMID: 34121337 Free PMC article.
Cited by
-
Navigating the Landscape of Hydrocortisone Administration in Septic Shock: Current Concepts and Future Directions.Cureus. 2023 Dec 3;15(12):e49870. doi: 10.7759/cureus.49870. eCollection 2023 Dec. Cureus. 2023. PMID: 38169849 Free PMC article. Review.
-
A Retrospective Study of Dexamethasone, Remdesivir, and Baricitinib in Severe COVID-19.Can J Infect Dis Med Microbiol. 2022 Jul 6;2022:9209618. doi: 10.1155/2022/9209618. eCollection 2022. Can J Infect Dis Med Microbiol. 2022. PMID: 35845297 Free PMC article.
-
Multi-Residue Detection of Eight Glucocorticoids by Nano-Au/Fluticasone Propionate Electrochemical Immunosensor.Molecules. 2023 Sep 14;28(18):6619. doi: 10.3390/molecules28186619. Molecules. 2023. PMID: 37764395 Free PMC article.
-
Extract of Cimicifuga racemosa (L.) Nutt protects ovarian follicle reserve of mice against in vitro deleterious effects of dexamethasone.Braz J Med Biol Res. 2023 Sep 22;56:e12811. doi: 10.1590/1414-431X2023e12811. eCollection 2023. Braz J Med Biol Res. 2023. PMID: 37792779 Free PMC article.
-
Immune and ionic mechanisms mediating the effect of dexamethasone in severe COVID-19.Front Immunol. 2023 Mar 24;14:1143350. doi: 10.3389/fimmu.2023.1143350. eCollection 2023. Front Immunol. 2023. PMID: 37033961 Free PMC article.
References
-
- McNeill W.H. Plagues and Peoples. Archor Press; New York, NY, USA: 1976.
-
- WHO Health Topics: Severe Acute Respiratory Syndrome. [(accessed on 30 April 2021)]; Available online: http://www.emro.who.int/health-topics/severe-acute-respiratory-syndrome/
-
- WHO Health Topics: Middle East Respiratory Syndrome. [(accessed on 30 April 2021)]; Available online: http://www.emro.who.int/health-topics/mers-cov/mers-outbreaks.html.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

