Psychoactive Drugs Induce the SOS Response and Shiga Toxin Production in Escherichia coli
- PMID: 34201801
- PMCID: PMC8309737
- DOI: 10.3390/toxins13070437
Psychoactive Drugs Induce the SOS Response and Shiga Toxin Production in Escherichia coli
Abstract
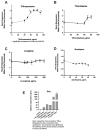
Several classes of non-antibiotic drugs, including psychoactive drugs, proton-pump inhibitors (PPIs), non-steroidal anti-inflammatory drugs (NSAIDs), and others, appear to have strong antimicrobial properties. We considered whether psychoactive drugs induce the SOS response in E. coli bacteria and, consequently, induce Shiga toxins in Shiga-toxigenic E. coli (STEC). We measured the induction of an SOS response using a recA-lacZ E. coli reporter strain, as RecA is an early, reliable, and quantifiable marker for activation of the SOS stress response pathway. We also measured the production and release of Shiga toxin 2 (Stx2) from a classic E. coli O157:H7 strain, derived from a food-borne outbreak due to spinach. Some, but not all, serotonin selective reuptake inhibitors (SSRIs) and antipsychotic drugs induced an SOS response. The use of SSRIs is widespread and increasing; thus, the use of these antidepressants could account for some cases of hemolytic-uremic syndrome due to STEC and is not attributable to antibiotic administration. SSRIs could have detrimental effects on the normal intestinal microbiome in humans. In addition, as SSRIs are resistant to environmental breakdown, they could have effects on microbial communities, including aquatic ecosystems, long after they have left the human body.
Keywords: RecA; Shiga-toxigenic E. coli; enterohemorrhagic E. coli; hemolytic-uremic syndrome; hypermutation; phenothiazines; serotonin selective reuptake inhibitors.
Conflict of interest statement
The authors have no financial or other conflict of interest to declare.
Figures



Similar articles
-
Induction of Shiga Toxin-Encoding Prophage by Abiotic Environmental Stress in Food.Appl Environ Microbiol. 2017 Sep 15;83(19):e01378-17. doi: 10.1128/AEM.01378-17. Print 2017 Oct 1. Appl Environ Microbiol. 2017. PMID: 28778890 Free PMC article.
-
Effect of rifampicin and gentamicin on Shiga toxin 2 expression level and the SOS response in Escherichia coli O104:H4.Foodborne Pathog Dis. 2015 Jan;12(1):47-55. doi: 10.1089/fpd.2014.1824. Epub 2014 Nov 10. Foodborne Pathog Dis. 2015. PMID: 25383748
-
Effect of antibiotics on cellular stress generated in Shiga toxin-producing Escherichia coli O157:H7 and non-O157 biofilms.Toxicol In Vitro. 2015 Oct;29(7):1692-700. doi: 10.1016/j.tiv.2015.06.025. Epub 2015 Jun 27. Toxicol In Vitro. 2015. PMID: 26130220
-
Advances in pathogenesis and therapy of hemolytic uremic syndrome caused by Shiga toxin-2.IUBMB Life. 2013 Oct;65(10):827-35. doi: 10.1002/iub.1206. Epub 2013 Sep 6. IUBMB Life. 2013. PMID: 24014500 Review.
-
Shiga toxin-induced haemolytic uraemic syndrome and the role of antibiotics: a global overview.J Infect. 2019 Aug;79(2):75-94. doi: 10.1016/j.jinf.2019.05.018. Epub 2019 May 28. J Infect. 2019. PMID: 31150744 Review.
Cited by
-
Role of Extracellular DNA in Bacterial Response to SOS-Inducing Drugs.Antibiotics (Basel). 2023 Mar 24;12(4):649. doi: 10.3390/antibiotics12040649. Antibiotics (Basel). 2023. PMID: 37107011 Free PMC article.
-
Cranberry constituents prevent SOS-mediated filamentation of uropathogenic Escherichia coli.Infect Immun. 2025 May 13;93(5):e0060024. doi: 10.1128/iai.00600-24. Epub 2025 Apr 10. Infect Immun. 2025. PMID: 40208062 Free PMC article.
-
Repurposing Antidepressants and Phenothiazine Antipsychotics as Efflux Pump Inhibitors in Cancer and Infectious Diseases.Antibiotics (Basel). 2023 Jan 10;12(1):137. doi: 10.3390/antibiotics12010137. Antibiotics (Basel). 2023. PMID: 36671340 Free PMC article. Review.
-
Defects in DNA double-strand break repair resensitize antibiotic-resistant Escherichia coli to multiple bactericidal antibiotics.Microbiologyopen. 2022 Oct;11(5):e1316. doi: 10.1002/mbo3.1316. Microbiologyopen. 2022. PMID: 36314749 Free PMC article.
-
Rapid assembly of biofilms from DNA released by SOS-inducing drugs in enteric bacteria.Sci Rep. 2025 Apr 13;15(1):12711. doi: 10.1038/s41598-025-96943-2. Sci Rep. 2025. PMID: 40223123 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

