RGS14 Regulation of Post-Synaptic Signaling and Spine Plasticity in Brain
- PMID: 34201943
- PMCID: PMC8268017
- DOI: 10.3390/ijms22136823
RGS14 Regulation of Post-Synaptic Signaling and Spine Plasticity in Brain
Abstract
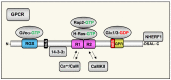
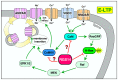
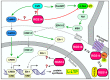
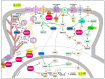
The regulator of G-protein signaling 14 (RGS14) is a multifunctional signaling protein that regulates post synaptic plasticity in neurons. RGS14 is expressed in the brain regions essential for learning, memory, emotion, and stimulus-induced behaviors, including the basal ganglia, limbic system, and cortex. Behaviorally, RGS14 regulates spatial and object memory, female-specific responses to cued fear conditioning, and environmental- and psychostimulant-induced locomotion. At the cellular level, RGS14 acts as a scaffolding protein that integrates G protein, Ras/ERK, and calcium/calmodulin signaling pathways essential for spine plasticity and cell signaling, allowing RGS14 to naturally suppress long-term potentiation (LTP) and structural plasticity in hippocampal area CA2 pyramidal cells. Recent proteomics findings indicate that RGS14 also engages the actomyosin system in the brain, perhaps to impact spine morphogenesis. Of note, RGS14 is also a nucleocytoplasmic shuttling protein, where its role in the nucleus remains uncertain. Balanced nuclear import/export and dendritic spine localization are likely essential for RGS14 neuronal functions as a regulator of synaptic plasticity. Supporting this idea, human genetic variants disrupting RGS14 localization also disrupt RGS14's effects on plasticity. This review will focus on the known and unexplored roles of RGS14 in cell signaling, physiology, disease and behavior.
Keywords: 14-3-3; G protein; H-Ras; RGS protein; RGS14; calcium; calmodulin; hippocampus; nucleocytoplasmic shuttling; synaptic plasticity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

