Efficient Heat Shock Response Affects Hyperthermia-Induced Radiosensitization in a Tumor Spheroid Control Probability Assay
- PMID: 34201993
- PMCID: PMC8269038
- DOI: 10.3390/cancers13133168
Efficient Heat Shock Response Affects Hyperthermia-Induced Radiosensitization in a Tumor Spheroid Control Probability Assay
Abstract
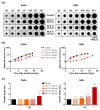
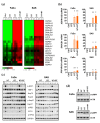
Hyperthermia (HT) combined with irradiation is a well-known concept to improve the curative potential of radiotherapy. Technological progress has opened new avenues for thermoradiotherapy, even for recurrent head and neck squamous cell carcinomas (HNSCC). Preclinical evaluation of the curative radiosensitizing potential of various HT regimens remains ethically, economically, and technically challenging. One key objective of our study was to refine an advanced 3-D assay setup for HT + RT research and treatment testing. For the first time, HT-induced radiosensitization was systematically examined in two differently radioresponsive HNSCC spheroid models using the unique in vitro "curative" analytical endpoint of spheroid control probability. We further investigated the cellular stress response mechanisms underlying the HT-related radiosensitization process with the aim to unravel the impact of HT-induced proteotoxic stress on the overall radioresponse. HT disrupted the proteome's thermal stability, causing severe proteotoxic stress. It strongly enhanced radiation efficacy and affected paramount survival and stress response signaling networks. Transcriptomics, q-PCR, and western blotting data revealed that HT + RT co-treatment critically triggers the heat shock response (HSR). Pre-treatment with chemical chaperones intensified the radiosensitizing effect, thereby suppressing HT-induced Hsp27 expression. Our data suggest that HT-induced radiosensitization is adversely affected by the proteotoxic stress response. Hence, we propose the inhibition of particular heat shock proteins as a targeting strategy to improve the outcome of combinatorial HT + RT.
Keywords: head and heck squamous cell carcinomas (HNSCC); heat shock proteins (Hsps); hyperthermia; proteotoxic stress; radiation therapy; spheroids.
Conflict of interest statement
In the past 5 years, M. Krause received funding for her research projects by IBA (2016), Merck KGaA (2014–2018 for preclinical study; 2018–2020 for clinical study), Medipan GmbH (2014–2018). She is involved in an ongoing publicly funded (German Federal Ministry of Education and Research) project with the companies Medipan, Attomol GmbH, GA Generic Assays GmbH, Gesellschaft für medizinische und wissenschaftliche genetische Analysen, Lipotype GmbH and PolyAn GmbH (2019–2021). For the present study, Krause confirms that none of the above mentioned funding sources were involved. The other authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

