Systematic Review on Optical Diagnosis of Early Gastrointestinal Neoplasia
- PMID: 34202001
- PMCID: PMC8269336
- DOI: 10.3390/jcm10132794
Systematic Review on Optical Diagnosis of Early Gastrointestinal Neoplasia
Abstract
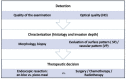
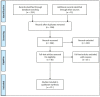
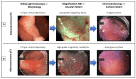
Background: Meticulous endoscopic characterization of gastrointestinal neoplasias (GN) is crucial to the clinical outcome. Hereby the indication and type of resection (endoscopically, en-bloc or piece-meal, or surgical resection) are determined. By means of established image-enhanced (IEE) and magnification endoscopy (ME) GN can be characterized in terms of malignancy and invasion depth. In this context, the statistical evidence and accuracy of these diagnostic procedures should be elucidated. Here, we present a systematic review of the literature.
Results: 21 Studies could be found which met the inclusion criteria. In clinical prospective trials and meta-analyses, the diagnostic accuracy of >90% for characterization of malignant neoplasms could be documented, if ME with IEE was used in squamous cell esophageal cancer, stomach, or colonic GN.
Conclusions: Currently, by means of optical diagnosis, today's gastrointestinal endoscopy is capable of determining the histological subtype, exact lateral spread, and depth of invasion of a lesion. The prerequisites for this are an exact knowledge of the anatomical structures, the endoscopic classifications based on them, and a systematic learning process, which can be supported by training courses. More prospective clinical studies are required, especially in the field of Barrett's esophagus and duodenal neoplasia.
Keywords: chromoendoscopy; endoscopy; invasion depth; magnification endoscopy; neoplasia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Vleugels J.L.A., Koens L., Dijkgraaf M.G.W., Houwen B., Hazewinkel Y., Fockens P., Dekker E. on behalf of the DISCOUNT study group. Suboptimal endoscopic cancer recognition in colorectal lesions in a national bowel screening programme. Gut. 2020;69:977–980. doi: 10.1136/gutjnl-2018-316882. - DOI - PMC - PubMed
-
- Atkinson N.S.S., Ket S., Bassett P., Aponte D., De Aguiar S., Gupta N., Horimatsu T., Ikematsu H., Inoue T., Kaltenbach T., et al. Narrow-band imaging for detection of neoplasia at colonoscopy: A meta-analysis of data from indi vidual patients in randomized controlled trials. Gastroenterology. 2019;157:462–471. doi: 10.1053/j.gastro.2019.04.014. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

