Early Aberrant Angiogenesis Due to Elastic Fiber Fragmentation in Aortic Valve Disease
- PMID: 34202041
- PMCID: PMC8303641
- DOI: 10.3390/jcdd8070075
Early Aberrant Angiogenesis Due to Elastic Fiber Fragmentation in Aortic Valve Disease
Abstract
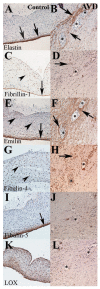
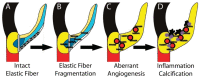
Elastic fiber fragmentation (EFF) is a hallmark of aortic valve disease (AVD), and neovascularization has been identified as a late finding related to inflammation. We sought to characterize the relationship between early EFF and aberrant angiogenesis. To examine disease progression, regional anatomy and pathology of aortic valve tissue were assessed using histochemistry, immunohistochemistry, and electron microscopy from early-onset (<40 yo) and late-onset (≥40 yo) non-syndromic AVD specimens. To assess the effects of EFF on early AVD processes, valve tissue from Williams and Marfan syndrome patients was also analyzed. Bicuspid aortic valve was more common in early-onset AVD, and cardiovascular comorbidities were more common in late-onset AVD. Early-onset AVD specimens demonstrated angiogenesis without inflammation or atherosclerosis. A distinct pattern of elastic fiber components surrounded early-onset AVD neovessels, including increased emilin-1 and decreased fibulin-5. Different types of EFF were present in Williams syndrome (WS) and Marfan syndrome (MFS) aortic valves; WS but not MFS aortic valves demonstrated angiogenesis. Aberrant angiogenesis occurs in early-onset AVD in the absence of inflammation, implicating EFF. Elucidation of underlying mechanisms may inform the development of new pharmacologic treatments.
Keywords: angiogenesis; aortic root; elastic fiber; heart valves; pediatrics.
Conflict of interest statement
The authors have no disclosure to make.
Figures



Similar articles
-
TGF-β mediates early angiogenesis and latent fibrosis in an Emilin1-deficient mouse model of aortic valve disease.Dis Model Mech. 2014 Aug;7(8):987-96. doi: 10.1242/dmm.015255. Dis Model Mech. 2014. PMID: 25056700 Free PMC article.
-
Inhibition of MAPK-Erk pathway in vivo attenuates aortic valve disease processes in Emilin1-deficient mouse model.Physiol Rep. 2017 Mar;5(5):e13152. doi: 10.14814/phy2.13152. Physiol Rep. 2017. PMID: 28270590 Free PMC article.
-
Maladaptive matrix remodeling and regional biomechanical dysfunction in a mouse model of aortic valve disease.Matrix Biol. 2012 Apr;31(3):197-205. doi: 10.1016/j.matbio.2012.01.001. Epub 2012 Jan 12. Matrix Biol. 2012. PMID: 22265892 Free PMC article.
-
Cardiovascular characteristics in Marfan syndrome and their relation to the genotype.Verh K Acad Geneeskd Belg. 2009;71(6):335-71. Verh K Acad Geneeskd Belg. 2009. PMID: 20232788 Review.
-
Advances in the treatment of aortic valve disease: is it time for companion diagnostics?Curr Opin Pediatr. 2014 Oct;26(5):546-52. doi: 10.1097/MOP.0000000000000137. Curr Opin Pediatr. 2014. PMID: 25089943 Free PMC article. Review.
Cited by
-
Sex-specific role of galectin-3 in aortic stenosis.Biol Sex Differ. 2023 Oct 24;14(1):72. doi: 10.1186/s13293-023-00556-1. Biol Sex Differ. 2023. PMID: 37875993 Free PMC article.
-
Characterization of the sex-specific pattern of angiogenesis and lymphangiogenesis in aortic stenosis.Front Cardiovasc Med. 2022 Sep 12;9:971802. doi: 10.3389/fcvm.2022.971802. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36172587 Free PMC article.
-
Focusing on the Native Matrix Proteins in Calcific Aortic Valve Stenosis.JACC Basic Transl Sci. 2023 Mar 29;8(8):1028-1039. doi: 10.1016/j.jacbts.2023.01.009. eCollection 2023 Aug. JACC Basic Transl Sci. 2023. PMID: 37719438 Free PMC article. Review.
References
-
- Go A.S., Mozaffarian D., Roger V.L., Benjamin E.J., Berry J.D., Blaha M.J., Dai S., Ford E.S., Fox C.S., Franco S., et al. Heart Disease and Stroke Statistics—2014 Update: A Report From the American Heart Association. Circulation. 2013;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. - DOI - PMC - PubMed
-
- Bonow R.O., Carabello B.A., Chatterjee K., de Leon A.C., Faxon D.P., Freed M.D., Gaasch W.H., Lytle B.W., Nishimura R.A., O’Gara P.T., et al. 2008 Focused Update Incorporated Into the ACC/AHA 2006 Guidelines for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease) Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2008;52:e1–e142. doi: 10.1016/j.jacc.2008.05.007. - DOI - PubMed
-
- Rajamannan N.M., Evans F.J., Aikawa E., Grande-Allen K.J., Demer L.L., Heistad D.D., Simmons C.A., Masters K.S., Mathieu P., O’Brien K.D., et al. Calcific aortic valve disease: Not simply a degenerative process: A review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Circulation. 2011;124:1783–1791. doi: 10.1161/CIRCULATIONAHA.110.006767. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

