Computational Modeling of Spinal Locomotor Circuitry in the Age of Molecular Genetics
- PMID: 34202085
- PMCID: PMC8267724
- DOI: 10.3390/ijms22136835
Computational Modeling of Spinal Locomotor Circuitry in the Age of Molecular Genetics
Abstract
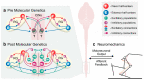
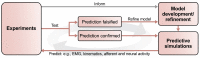
Neuronal circuits in the spinal cord are essential for the control of locomotion. They integrate supraspinal commands and afferent feedback signals to produce coordinated rhythmic muscle activations necessary for stable locomotion. For several decades, computational modeling has complemented experimental studies by providing a mechanistic rationale for experimental observations and by deriving experimentally testable predictions. This symbiotic relationship between experimental and computational approaches has resulted in numerous fundamental insights. With recent advances in molecular and genetic methods, it has become possible to manipulate specific constituent elements of the spinal circuitry and relate them to locomotor behavior. This has led to computational modeling studies investigating mechanisms at the level of genetically defined neuronal populations and their interactions. We review literature on the spinal locomotor circuitry from a computational perspective. By reviewing examples leading up to and in the age of molecular genetics, we demonstrate the importance of computational modeling and its interactions with experiments. Moving forward, neuromechanical models with neuronal circuitry modeled at the level of genetically defined neuronal populations will be required to further unravel the mechanisms by which neuronal interactions lead to locomotor behavior.
Keywords: central pattern generator; computational modeling; interneurons; neuronal control of locomotion; sensory feedback; spinal cord.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Modelling spinal circuitry involved in locomotor pattern generation: insights from deletions during fictive locomotion.J Physiol. 2006 Dec 1;577(Pt 2):617-39. doi: 10.1113/jphysiol.2006.118703. Epub 2006 Sep 28. J Physiol. 2006. PMID: 17008376 Free PMC article.
-
Organization of flexor-extensor interactions in the mammalian spinal cord: insights from computational modelling.J Physiol. 2016 Nov 1;594(21):6117-6131. doi: 10.1113/JP272437. Epub 2016 Jul 21. J Physiol. 2016. PMID: 27292055 Free PMC article.
-
Neuronal activity in the isolated mouse spinal cord during spontaneous deletions in fictive locomotion: insights into locomotor central pattern generator organization.J Physiol. 2012 Oct 1;590(19):4735-59. doi: 10.1113/jphysiol.2012.240895. Epub 2012 Aug 6. J Physiol. 2012. PMID: 22869012 Free PMC article.
-
Dorsally derived spinal interneurons in locomotor circuits.Ann N Y Acad Sci. 2013 Mar;1279:32-42. doi: 10.1111/j.1749-6632.2012.06801.x. Ann N Y Acad Sci. 2013. PMID: 23531000 Review.
-
Origin and circuitry of spinal locomotor interneurons generating different speeds.Curr Opin Neurobiol. 2018 Dec;53:16-21. doi: 10.1016/j.conb.2018.04.024. Epub 2018 May 4. Curr Opin Neurobiol. 2018. PMID: 29733915 Review.
Cited by
-
A Whole-Body Musculoskeletal Model of the Mouse.IEEE Access. 2021;9:163861-163881. doi: 10.1109/access.2021.3133078. Epub 2021 Dec 6. IEEE Access. 2021. PMID: 35211364 Free PMC article.
-
The role of V3 neurons in speed-dependent interlimb coordination during locomotion in mice.Elife. 2022 Apr 27;11:e73424. doi: 10.7554/eLife.73424. Elife. 2022. PMID: 35476640 Free PMC article.
-
Spinal control of locomotion before and after spinal cord injury.bioRxiv [Preprint]. 2023 Jun 1:2023.03.22.533794. doi: 10.1101/2023.03.22.533794. bioRxiv. 2023. Update in: Exp Neurol. 2023 Oct;368:114496. doi: 10.1016/j.expneurol.2023.114496. PMID: 36993490 Free PMC article. Updated. Preprint.
-
The spinal premotor network driving scratching flexor and extensor alternation.bioRxiv [Preprint]. 2025 Jan 8:2025.01.08.631866. doi: 10.1101/2025.01.08.631866. bioRxiv. 2025. Update in: Cell Rep. 2025 Jun 24;44(6):115845. doi: 10.1016/j.celrep.2025.115845. PMID: 39829804 Free PMC article. Updated. Preprint.
-
Spinal control of locomotion before and after spinal cord injury.Exp Neurol. 2023 Oct;368:114496. doi: 10.1016/j.expneurol.2023.114496. Epub 2023 Jul 25. Exp Neurol. 2023. PMID: 37499972 Free PMC article.
References
-
- Grillner S. Control of Locomotion in Bipeds, Tetrapods, and Fish. In: Brooks V.B., editor. Handbook of Physiology: The Nervous System Volume 2: Motor Control. American Physiology Society; Bethesda, MD, USA: 1981. pp. 1179–1236.
-
- Kiehn O., Dougherty K. Locomotion: Circuits and Physiology. In: Pfaff D.W., Volkow N.D., editors. Neuroscience in the 21st Century: From Basic to Clinical. Springer; New York, NY, USA: 2016. pp. 1337–1365.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

