How Do Enveloped Viruses Exploit the Secretory Proprotein Convertases to Regulate Infectivity and Spread?
- PMID: 34202098
- PMCID: PMC8310232
- DOI: 10.3390/v13071229
How Do Enveloped Viruses Exploit the Secretory Proprotein Convertases to Regulate Infectivity and Spread?
Abstract
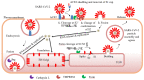
Inhibition of the binding of enveloped viruses surface glycoproteins to host cell receptor(s) is a major target of vaccines and constitutes an efficient strategy to block viral entry and infection of various host cells and tissues. Cellular entry usually requires the fusion of the viral envelope with host plasma membranes. Such entry mechanism is often preceded by "priming" and/or "activation" steps requiring limited proteolysis of the viral surface glycoprotein to expose a fusogenic domain for efficient membrane juxtapositions. The 9-membered family of Proprotein Convertases related to Subtilisin/Kexin (PCSK) serine proteases (PC1, PC2, Furin, PC4, PC5, PACE4, PC7, SKI-1/S1P, and PCSK9) participate in post-translational cleavages and/or regulation of multiple secretory proteins. The type-I membrane-bound Furin and SKI-1/S1P are the major convertases responsible for the processing of surface glycoproteins of enveloped viruses. Stefan Kunz has considerably contributed to define the role of SKI-1/S1P in the activation of arenaviruses causing hemorrhagic fever. Furin was recently implicated in the activation of the spike S-protein of SARS-CoV-2 and Furin-inhibitors are being tested as antivirals in COVID-19. Other members of the PCSK-family are also implicated in some viral infections, such as PCSK9 in Dengue. Herein, we summarize the various functions of the PCSKs and present arguments whereby their inhibition could represent a powerful arsenal to limit viral infections causing the present and future pandemics.
Keywords: COVID-19; Furin; PCSK9; SARS-CoV-2; SKI-1/S1P; enveloped virus; pandemic; proprotein convertases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Dobson A.P., Carper E.R. Infectious Diseases and Human Population History: Throughout history the establishment of disease has been a side effect of the growth of civilization. Bioscience. 1996;46:115–126. doi: 10.2307/1312814. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

