Antioxidant Properties of Ergosterol and Its Role in Yeast Resistance to Oxidation
- PMID: 34202105
- PMCID: PMC8300696
- DOI: 10.3390/antiox10071024
Antioxidant Properties of Ergosterol and Its Role in Yeast Resistance to Oxidation
Abstract
Although the functions and structural roles of sterols have been the subject of numerous studies, the reasons for the diversity of sterols in the different eukaryotic kingdoms remain unclear. It is thought that the specificity of sterols is linked to unidentified supplementary functions that could enable organisms to be better adapted to their environment. Ergosterol is accumulated by late branching fungi that encounter oxidative perturbations in their interfacial habitats. Here, we investigated the antioxidant properties of ergosterol using in vivo, in vitro, and in silico approaches. The results showed that ergosterol is involved in yeast resistance to tert-butyl hydroperoxide and protects lipids against oxidation in liposomes. A computational study based on quantum chemistry revealed that this protection could be related to its antioxidant properties operating through an electron transfer followed by a proton transfer mechanism. This study demonstrates the antioxidant role of ergosterol and proposes knowledge elements to explain the specific accumulation of this sterol in late branching fungi. Ergosterol, as a natural antioxidant molecule, could also play a role in the incompletely understood beneficial effects of some mushrooms on health.
Keywords: antioxidant; lipids; oxidation; plasma membrane; sterol; yeast.
Conflict of interest statement
Authors declare that they have no conflict of interest.
Figures

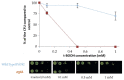
 ) and erg6Δ (
) and erg6Δ ( ) strains of S. cerevisiae are grown for 48 h at 25 °C in a yeast extract–peptone–dextrose (YPD) medium supplemented with various concentrations of t-BOOH (0, 0.1, 0.5, or 1 mM). Cells were spotted at different ten-fold dilutions (from 10−1 to 10−4) from the initial suspension at OD600 nm = 0.5. Data are presented as mean values ± standard deviation of three independent experiments.
) strains of S. cerevisiae are grown for 48 h at 25 °C in a yeast extract–peptone–dextrose (YPD) medium supplemented with various concentrations of t-BOOH (0, 0.1, 0.5, or 1 mM). Cells were spotted at different ten-fold dilutions (from 10−1 to 10−4) from the initial suspension at OD600 nm = 0.5. Data are presented as mean values ± standard deviation of three independent experiments.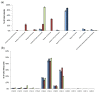
 ), RH448 WT (
), RH448 WT ( ), erg6Δ (
), erg6Δ ( ), and erg2Δerg6Δ (
), and erg2Δerg6Δ ( ) strains of S. cerevisiae. (a) Analysis of the sterol composition and the relative abundance. (b) Analysis of the fatty acid composition and the relative abundance. Vertical bars represent standard deviation.
) strains of S. cerevisiae. (a) Analysis of the sterol composition and the relative abundance. (b) Analysis of the fatty acid composition and the relative abundance. Vertical bars represent standard deviation.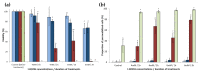
 ), RH448 WT (
), RH448 WT ( ), erg6Δ (
), erg6Δ ( ), and erg2Δerg6Δ (
), and erg2Δerg6Δ ( ) strains of S. cerevisiae were washed, adjusted to a concentration corresponding to DO600 nm = 0.5, and placed in PBS containing t-BOOH (4 or 6 mM) for 1 or 2 h. (a) After treatment, the cells were washed and spotted at different ten-fold dilutions (from 10−1 to 10−4) to assess the cell viability. (b) Plasma membrane integrity was assessed by PI staining of cells and flow cytometry analysis. Data are presented as mean values ± standard deviation of three independent experiments. ANOVA was performed on R v3.6.1 software and if it was significant (p < 0.01), Tukey’s HSD (Honest Significant Difference) test was performed to observe significant differences among conditions. Letters a, b, c, d, and e represent significantly different groups.
) strains of S. cerevisiae were washed, adjusted to a concentration corresponding to DO600 nm = 0.5, and placed in PBS containing t-BOOH (4 or 6 mM) for 1 or 2 h. (a) After treatment, the cells were washed and spotted at different ten-fold dilutions (from 10−1 to 10−4) to assess the cell viability. (b) Plasma membrane integrity was assessed by PI staining of cells and flow cytometry analysis. Data are presented as mean values ± standard deviation of three independent experiments. ANOVA was performed on R v3.6.1 software and if it was significant (p < 0.01), Tukey’s HSD (Honest Significant Difference) test was performed to observe significant differences among conditions. Letters a, b, c, d, and e represent significantly different groups.
 ), with a zymosterol/phospholipid molar ratio of 1/3 (
), with a zymosterol/phospholipid molar ratio of 1/3 ( ), with a cholesta-5,7,24-trienol/phospholipid molar ratio of 1/3 (
), with a cholesta-5,7,24-trienol/phospholipid molar ratio of 1/3 ( ), or with an ergosterol/phospholipid molar ratio of 1/3 (
), or with an ergosterol/phospholipid molar ratio of 1/3 ( ). Control experiments were performed with lipososmes with a tocopherol/phospholipid molar ratio of 1/3 (
). Control experiments were performed with lipososmes with a tocopherol/phospholipid molar ratio of 1/3 ( ). (a) Phospholipid oxidation induced by cumene hydroperoxide and haemin was assessed by the oxidation kinetics of BP-C11 in liposomes with different sterol compositions. Oxidation was expressed as the intensity ratio (I520 nm)oxidized/(I590 nm)non-oxidized. Oxidation was started at t = 0 s. (b) Fluidity of liposomes was assessed by steady-state DPH fluorescence anisotropy measurement as a function of temperature in the range 4–48 °C. The results are presented with error bars corresponding to the standard deviation calculated from three repeated experiments.
). (a) Phospholipid oxidation induced by cumene hydroperoxide and haemin was assessed by the oxidation kinetics of BP-C11 in liposomes with different sterol compositions. Oxidation was expressed as the intensity ratio (I520 nm)oxidized/(I590 nm)non-oxidized. Oxidation was started at t = 0 s. (b) Fluidity of liposomes was assessed by steady-state DPH fluorescence anisotropy measurement as a function of temperature in the range 4–48 °C. The results are presented with error bars corresponding to the standard deviation calculated from three repeated experiments.

Similar articles
-
Ergosterol biosynthesis: a fungal pathway for life on land?Evolution. 2012 Sep;66(9):2961-8. doi: 10.1111/j.1558-5646.2012.01667.x. Epub 2012 May 14. Evolution. 2012. PMID: 22946816
-
Nature of sterols affects plasma membrane behavior and yeast survival during dehydration.Biochim Biophys Acta. 2011 Jun;1808(6):1520-8. doi: 10.1016/j.bbamem.2010.11.012. Epub 2010 Nov 13. Biochim Biophys Acta. 2011. PMID: 21081111
-
Influence of sterol structure on yeast plasma membrane properties.Biochim Biophys Acta. 1985 Mar 14;813(2):313-20. doi: 10.1016/0005-2736(85)90247-0. Biochim Biophys Acta. 1985. PMID: 3882148
-
Ergosterol Turnover in Yeast: An Interplay between Biosynthesis and Transport.Biochemistry (Mosc). 2019 Apr;84(4):346-357. doi: 10.1134/S0006297919040023. Biochemistry (Mosc). 2019. PMID: 31228926 Review.
-
Mechanisms of sterol uptake and transport in yeast.J Steroid Biochem Mol Biol. 2012 Mar;129(1-2):70-8. doi: 10.1016/j.jsbmb.2010.11.014. Epub 2010 Dec 8. J Steroid Biochem Mol Biol. 2012. PMID: 21145395 Review.
Cited by
-
Potential antioxidant and antiradical agents from Allium ascalonicum: Superoxide dismutase and density functional theory in silico studies.J Adv Pharm Technol Res. 2024 Jul-Sep;15(3):171-176. doi: 10.4103/japtr.japtr_525_23. Epub 2024 Jul 22. J Adv Pharm Technol Res. 2024. PMID: 39290541 Free PMC article.
-
Integrated Transcriptomics and Metabolomics Analysis Reveal the Regulatory Mechanisms Underlying Sodium Butyrate-Induced Carotenoid Biosynthesis in Rhodotorula glutinis.J Fungi (Basel). 2024 Apr 27;10(5):320. doi: 10.3390/jof10050320. J Fungi (Basel). 2024. PMID: 38786675 Free PMC article.
-
Ergosterol Protects Canine MDCK Cells from Gentamicin-Induced Damage by Modulating Autophagy and Apoptosis.Metabolites. 2025 Jun 5;15(6):373. doi: 10.3390/metabo15060373. Metabolites. 2025. PMID: 40559397 Free PMC article.
-
Expanding the CRISPR Toolbox for Engineering Lycopene Biosynthesis in Corynebacterium glutamicum.Microorganisms. 2024 Apr 16;12(4):803. doi: 10.3390/microorganisms12040803. Microorganisms. 2024. PMID: 38674747 Free PMC article.
-
Evaluation of Bioactive Compounds, Antioxidant Activity, and Anticancer Potential of Wild Ganoderma lucidum Extracts from High-Altitude Regions of Nepal.Curr Issues Mol Biol. 2025 Aug 5;47(8):624. doi: 10.3390/cimb47080624. Curr Issues Mol Biol. 2025. PMID: 40864778 Free PMC article.
References
-
- Tanret C. Sur Un Nouveau Principle Immediate de l’ergot Deseigle Ergosterine. CR Seances Acad. Sci. 1889;108:98–100.
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

