Extracellular Vesicles Are More Potent Than Adipose Mesenchymal Stromal Cells to Exert an Anti-Fibrotic Effect in an In Vitro Model of Systemic Sclerosis
- PMID: 34202139
- PMCID: PMC8269376
- DOI: 10.3390/ijms22136837
Extracellular Vesicles Are More Potent Than Adipose Mesenchymal Stromal Cells to Exert an Anti-Fibrotic Effect in an In Vitro Model of Systemic Sclerosis
Abstract
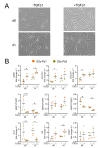
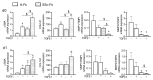
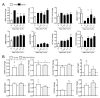
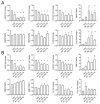
Systemic sclerosis (SSc) is a complex disorder resulting from dysregulated interactions between the three main pathophysiological axes: fibrosis, immune dysfunction, and vasculopathy, with no specific treatment available to date. Adipose tissue-derived mesenchymal stromal cells (ASCs) and their extracellular vesicles (EVs) have proved efficacy in pre-clinical murine models of SSc. However, their precise action mechanism is still not fully understood. Because of the lack of availability of fibroblasts isolated from SSc patients (SSc-Fb), our aim was to determine whether a TGFβ1-induced model of human myofibroblasts (Tβ-Fb) could reproduce the characteristics of SSc-Fb and be used to evaluate the anti-fibrotic function of ASCs and their EVs. We found out that Tβ-Fb displayed the main morphological and molecular features of SSc-Fb, including the enlarged hypertrophic morphology and expression of several markers associated with the myofibroblastic phenotype. Using this model, we showed that ASCs were able to regulate the expression of most myofibroblastic markers on Tβ-Fb and SSc-Fb, but only when pre-stimulated with TGFβ1. Of interest, ASC-derived EVs were more effective than parental cells for improving the myofibroblastic phenotype. In conclusion, we provided evidence that Tβ-Fb are a relevant model to mimic the main characteristics of SSc fibroblasts and investigate the mechanism of action of ASCs. We further reported that ASC-EVs are more effective than parental cells suggesting that the TGFβ1-induced pro-fibrotic environment may alter the function of ASCs.
Keywords: TGFβ1; anti-fibrotic; extracellular vesicles; mesenchymal stem cells; systemic sclerosis.
Conflict of interest statement
The authors disclose any financial or personal conflicts of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

