Assessment of the Suitability of Melilotus officinalis for Phytoremediation of Soil Contaminated with Petroleum Hydrocarbons (TPH and PAH), Zn, Pb and Cd Based on Toxicological Tests
- PMID: 34202316
- PMCID: PMC8309879
- DOI: 10.3390/toxics9070148
Assessment of the Suitability of Melilotus officinalis for Phytoremediation of Soil Contaminated with Petroleum Hydrocarbons (TPH and PAH), Zn, Pb and Cd Based on Toxicological Tests
Abstract
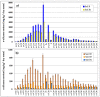
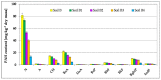
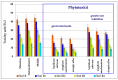
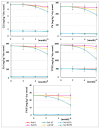
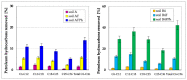
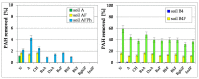
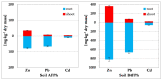
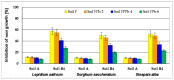
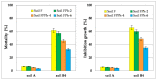
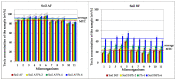
The article presents issues related to the possibility of using toxicological tests as a tool to monitor the progress of soil treatment contaminated with petroleum substances (TPH, PAH), Zn, Pb and Cd in bio-phytoremediation processes. In order to reduce the high content of petroleum pollutants (TPH = 56,371 mg kg-1 dry mass, PAH = 139.3 mg kg-1 dry mass), the technology of stepwise soil treatment was applied, including basic bioremediation and inoculation with biopreparations based of indigenous non-pathogenic species of bacteria, fungi and yeasts. As a result of basic bioremediation in laboratory conditions (ex-situ method), the reduction of petroleum pollutants TPH by 33.9% and PAH by 9.5% was achieved. The introduction of inoculation with biopraparation-1 prepared on the basis of non-pathogenic species of indigenous bacteria made it possible to reduce the TPH content by 86.3%, PAH by 40.3%. The use of a biopreparation-1 enriched with indigenous non-pathogenic species of fungi and yeasts in the third series of inoculation increased to an increase in the degree of biodegradation of aliphatic hydrocarbons with long carbon chains and PAH by a further 28.9%. In the next stage of soil treatment after biodegradation processes, which was characterized by an increased content of heavy metals (Zn, Pb, Cd) and naphthalene, chrysene, benzo(a)anthracene and benzo(ghi)perylene belonging to polycyclic aromatic hydrocarbons, phytoremediation with the use of Melilotus officinalis was applied. After the six-month phytoremediation process, the following was achieved: Zn content by 25.1%, Pb by 27.9%, Cd by 23.2% and TPH by 42.2% and PAH by 49.9%. The rate of removal of individual groups of hydrocarbons was in the decreasing order: C12-C18 > C6-C12 > C18-C25 > C25-C36. PAHs tended to be removed in the following order: chrysene > naphthalene > benzo(a)anthracene > benzo(ghi)perylene. The TF and BCF coefficients were calculated to assess the capacity of M. officinalis to accumulate metal in tissues, uptake from soil and transfer from roots to shoots. The values of TF translocation coefficients were, respectively, for Zn (0.44), Pb (0.12), Cd (0.40). The calculated BCF concentration factors (BCFroots > BCFshoots) show that heavy metals taken up by M. officinalis are mainly accumulated in the root tissues in the following order Zn > Pb > Cd, revealing a poor metal translocation from the root to the shoots. This process was carried out in laboratory conditions for a period of 6 months. The process of phytoremediation of contaminated soil using M. officinalis assisted with fertilization was monitored by means of toxicological tests: Microtox, Ostracodtoxkit FTM, MARA and PhytotoxkitTM. The performed phytotoxicity tests have indicated variable sensitivity of the tested plants on contaminants occurring in the studied soils, following the sequence: Lepidium sativum < Sorghum saccharatum < Sinapis alba. The sensitivity of toxicological tests was comparable and increased in the order: MARA < Ostracodtoxkit FTM < Microtox. The results of the toxicological monitoring as a function of the time of soil treatment, together with chemical analyses determining the content of toxicants in soil and biomass M. officinalis, clearly confirmed the effectiveness of the applied concept of bioremediation of soils contaminated with zinc, lead and cadmium in the presence of petroleum hydrocarbons.
Keywords: Melilotus officinalis; biodegradation; heavy metals; phytoremediation; polycyclic aromatic hydrocarbons; soil; total petroleum hydrocarbons; toxicological tests.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Application of Festuca arundinacea in phytoremediation of soils contaminated with Pb, Ni, Cd and petroleum hydrocarbons.Ecotoxicol Environ Saf. 2020 May;194:110409. doi: 10.1016/j.ecoenv.2020.110409. Epub 2020 Mar 7. Ecotoxicol Environ Saf. 2020. PMID: 32155481
-
Oil-Contaminated Soil Remediation with Biodegradation by Autochthonous Microorganisms and Phytoremediation by Maize (Zea mays).Molecules. 2023 Aug 17;28(16):6104. doi: 10.3390/molecules28166104. Molecules. 2023. PMID: 37630356 Free PMC article.
-
Synergistic effect of pyrene and heavy metals (Zn, Pb, and Cd) on phytoremediation potential of Medicago sativa L. (alfalfa) in multi-contaminated soil.Environ Sci Pollut Res Int. 2024 Mar;31(14):21012-21027. doi: 10.1007/s11356-024-32499-4. Epub 2024 Feb 21. Environ Sci Pollut Res Int. 2024. PMID: 38383928
-
Mitigation of petroleum-hydrocarbon-contaminated hazardous soils using organic amendments: A review.J Hazard Mater. 2021 Aug 15;416:125702. doi: 10.1016/j.jhazmat.2021.125702. Epub 2021 Mar 22. J Hazard Mater. 2021. PMID: 33866291 Review.
-
Biodegradation of polycyclic aromatic hydrocarbons: Using microbial bioelectrochemical systems to overcome an impasse.Environ Pollut. 2017 Dec;231(Pt 1):509-523. doi: 10.1016/j.envpol.2017.08.048. Epub 2017 Aug 29. Environ Pollut. 2017. PMID: 28841503 Review.
Cited by
-
Cultivation of Melilotus officinalis as a source of bioactive compounds in association with soil recovery practices.Front Plant Sci. 2023 Sep 12;14:1218594. doi: 10.3389/fpls.2023.1218594. eCollection 2023. Front Plant Sci. 2023. PMID: 37771488 Free PMC article.
-
Impact of Soil Pollution on Melliferous Plants.Toxics. 2022 May 9;10(5):239. doi: 10.3390/toxics10050239. Toxics. 2022. PMID: 35622652 Free PMC article.
-
Revitalization of Soil Contaminated by Petroleum Products Using Materials That Improve the Physicochemical and Biochemical Properties of the Soil.Molecules. 2024 Dec 11;29(24):5838. doi: 10.3390/molecules29245838. Molecules. 2024. PMID: 39769927 Free PMC article.
-
Heavy Metal Toxicity Effects on Plants.Toxics. 2022 Nov 23;10(12):715. doi: 10.3390/toxics10120715. Toxics. 2022. PMID: 36548548 Free PMC article.
-
Utilization of Indole Acetic Acid with Leucadendron rubrum and Rhododendron pulchrum for the Phytoremediation of Heavy Metals in the Artificial Soil Made of Municipal Sewage Sludge.Toxics. 2022 Dec 31;11(1):43. doi: 10.3390/toxics11010043. Toxics. 2022. PMID: 36668769 Free PMC article.
References
-
- Steliga T., Jakubowicz P., Kapusta P., Kluk D. Study on biodegradation of drilling wastes contaminated with petroleum hydrocarbons. Przemysł Chem. 2018;97:1666–1675. doi: 10.15199/62.2018.10.7. - DOI
-
- de Souza R.B., Maziviero T.G., Christofoletti C.A., Pinheiro T.G., Fontanetti C.S. Soil contamination with heavy metals and petroleum derivates: Impact on edaphic fauna and remediation strategies. Soil Process. Curr. Trends Qual. Assess. 2013;6:175–203. doi: 10.5772/52868. - DOI
-
- Moubasher H.A., Hegazy A.K., Mohamed N.H., Moustafa Y.M., Kabiel H.F., Hamad A.A. Phytoremediation of soils polluted with crude petroleum oil using Bassia scoparia and its associated rhizosphere microorganisms. Int. Biodeterior. Biodegrad. 2015;98:113–120. doi: 10.1016/j.ibiod.2014.11.019. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

