Perioperative Perfusion of Allografts with Anti-Human T-lymphocyte Globulin Does Not Improve Outcome Post Liver Transplantation-A Randomized Placebo-Controlled Trial
- PMID: 34202355
- PMCID: PMC8267618
- DOI: 10.3390/jcm10132816
Perioperative Perfusion of Allografts with Anti-Human T-lymphocyte Globulin Does Not Improve Outcome Post Liver Transplantation-A Randomized Placebo-Controlled Trial
Abstract
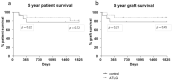
Due to the lack of suitable organs transplant surgeons have to accept unfavorable extended criteria donor (ECD) organs. Recently, we demonstrated that the perfusion of kidney organs with anti-human T-lymphocyte globulin (ATLG) prior to transplantation ameliorates ischemia-reperfusion injury (IRI). Here, we report on the results of perioperative ATLG perfusion in a randomized, single-blinded, placebo-controlled, feasibility trial (RCT) involving 30 liver recipients (LTx). Organs were randomly assigned for perfusion with ATLG/Grafalon® (AP) (n = 16) or saline only (control perfusion = CP) (n = 14) prior to implantation. The primary endpoint was defined as graft function reflected by aspartate transaminase (AST) values at day 7 post-transplantation (post-tx). With respect to the primary endpoint, no significant differences in AST levels were shown in the intervention group at day 7 (AP: 53.0 ± 21.3 mg/dL, CP: 59.7 ± 59.2 mg/dL, p = 0.686). Similarly, exploratory analysis of secondary clinical outcomes (e.g., patient survival) and treatment-specific adverse events revealed no differences between the study groups. Among liver transplant recipients, pre-operative organ perfusion with ATLG did not improve short-term outcomes, compared to those who received placebo perfusion. However, ATLG perfusion of liver grafts was proven to be a safe procedure without the occurrence of relevant adverse events.
Keywords: ATLG; liver transplantation; machine perfusion; organ perfusion; organ pretreatment; polyclonal antibody.
Conflict of interest statement
The authors declare no conflict of interest. The funders (Neovii Biotech GmbH) had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Graft Pre-conditioning by Peri-Operative Perfusion of Kidney Allografts With Rabbit Anti-human T-lymphocyte Globulin Results in Improved Kidney Graft Function in the Early Post-transplantation Period-a Prospective, Randomized Placebo-Controlled Trial.Front Immunol. 2018 Aug 24;9:1911. doi: 10.3389/fimmu.2018.01911. eCollection 2018. Front Immunol. 2018. PMID: 30197644 Free PMC article. Clinical Trial.
-
Long-term outcomes after standard graft-versus-host disease prophylaxis with or without anti-human-T-lymphocyte immunoglobulin in haemopoietic cell transplantation from matched unrelated donors: final results of a randomised controlled trial.Lancet Haematol. 2017 Jun;4(6):e293-e301. doi: 10.1016/S2352-3026(17)30081-9. Lancet Haematol. 2017. PMID: 28583289 Clinical Trial.
-
Efficacy of two different doses of rabbit anti-T-lymphocyte globulin to prevent graft-versus-host disease in children with haematological malignancies transplanted from an unrelated donor: a multicentre, randomised, open-label, phase 3 trial.Lancet Oncol. 2017 Aug;18(8):1126-1136. doi: 10.1016/S1470-2045(17)30417-5. Epub 2017 Jul 10. Lancet Oncol. 2017. PMID: 28705454 Clinical Trial.
-
Transplanting Marginal Organs in the Era of Modern Machine Perfusion and Advanced Organ Monitoring.Front Immunol. 2020 May 12;11:631. doi: 10.3389/fimmu.2020.00631. eCollection 2020. Front Immunol. 2020. PMID: 32477321 Free PMC article. Review.
-
Thymoglobulin and ischemia reperfusion injury in kidney and liver transplantation.Nephrol Dial Transplant. 2007 Sep;22 Suppl 8:viii54-viii60. doi: 10.1093/ndt/gfm651. Nephrol Dial Transplant. 2007. PMID: 17890265 Review.
References
-
- Jackson K.R., Motter J.D., Haugen C.E., Holscher C., Long J.J., Massie A.B., Philosophe B., Cameron A.M., Garonzik-Wang J., Segev D.L. Temporal trends in utilization and outcomes of steatotic donor livers in the United States. Am. J. Transplant. 2020;20:855–863. doi: 10.1111/ajt.15652. - DOI - PubMed
-
- Tedesco-Silva H.J., Mello Offerni J.C., Ayres Carneiro V., Ivani de Paula M., Neto E.D., Brambate Carvalhinho Lemos F., Requiao Moura L.R., Pacheco E.S.F.A., de Morais Cunha M.F., Francisco da Silva E., et al. Randomized Trial of Machine Perfusion Versus Cold Storage in Recipients of Deceased Donor Kidney Transplants With High Incidence of Delayed Graft Function. Transplant. Direct. 2017;3:e155. doi: 10.1097/TXD.0000000000000672. - DOI - PMC - PubMed
-
- Gallinat A., Amrillaeva V., Hoyer D.P., Kocabayoglu P., Benko T., Treckmann J.W., van Meel M., Samuel U., Minor T., Paul A. Reconditioning by end-ischemic hypothermic in-house machine perfusion: A promising strategy to improve outcome in expanded criteria donors kidney transplantation. Clin. Transplant. 2017;31 doi: 10.1111/ctr.12904. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

