The Role of Cholesterol in Amyloidogenic Substrate Binding to the γ-Secretase Complex
- PMID: 34202467
- PMCID: PMC8301813
- DOI: 10.3390/biom11070935
The Role of Cholesterol in Amyloidogenic Substrate Binding to the γ-Secretase Complex
Abstract
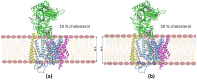
Alzheimer's disease is the most common progressive neurodegenerative disorder and is characterized by the presence of amyloid β (Aβ) plaques in the brain. The γ-secretase complex, which produces Aβ, is an intramembrane-cleaving protease consisting of four membrane proteins. In this paper we investigated the amyloidogenic fragments of amyloid precursor protein (substrates Aβ43 and Aβ45, leading to less amyloidogenic Aβ40 and more amyloidogenic Aβ42, respectively) docked to the binding site of presenilin, the catalytic subunit of γ-secretase. In total, we performed 9 μs of all-atom molecular dynamics simulations of the whole γ-secretase complex with both substrates in low (10%) and high (50%) concentrations of cholesterol in the membrane. We found that, at the high cholesterol level, the Aβ45 helix was statistically more flexible in the binding site of presenilin than Aβ43. An increase in the cholesterol concentration was also correlated with a higher flexibility of the Aβ45 helix, which suggests incompatibility between Aβ45 and the binding site of presenilin potentiated by a high cholesterol level. However, at the C-terminal part of Aβ45, the active site of presenilin was more compact in the case of a high cholesterol level, which could promote processing of this substrate. We also performed detailed mapping of the cholesterol binding sites at low and high cholesterol concentrations, which were independent of the typical cholesterol binding motifs.
Keywords: Alzheimer’s disease; amyloid precursor protein; cholesterol; membrane proteolysis; γ-secretase complex.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures











References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

