Blinding in Clinical Trials: Seeing the Big Picture
- PMID: 34202486
- PMCID: PMC8308085
- DOI: 10.3390/medicina57070647
Blinding in Clinical Trials: Seeing the Big Picture
Abstract
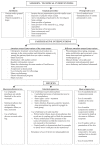
Blinding mitigates several sources of bias which, if left unchecked, can quantitively affect study outcomes. Blinding remains under-utilized, particularly in non-pharmaceutical clinical trials, but is often highly feasible through simple measures. Although blinding is generally viewed as an effective method by which to eliminate bias, blinding does also pose some inherent limitations, and it behooves clinicians and researchers to be aware of such caveats. This article will review general principles for blinding in clinical trials, including examples of useful blinding techniques for both pharmaceutical and non-pharmaceutical trials, while also highlighting the limitations and potential consequences of blinding. Appropriate reporting on blinding in trial protocols and manuscripts, as well as future directions for blinding research, will also be discussed.
Keywords: bias; blinding; clinical trials; double; single; triple.
Conflict of interest statement
Thomas F. Monaghan has no direct or indirect commercial incentive associated with publishing this article and certifies that all conflicts of interest relevant to the subject matter discussed in the manuscript are the following: Alan J. Wein has served as a consultant for Medtronic, Urovant, Antares, and Viveve, outside the submitted work. Karel Everaert is a consultant and lecturer for Medtronic and Ferring and reports institutional grants from Allergan, Ferring, Astellas, and Medtronic, outside the submitted work. The additional authors have nothing to disclose.
Figures

References
-
- Gresham G., Meinert J.L., Gresham A.G., Meinert C.L. Assessment of Trends in the Design, Accrual, and Completion of Trials Registered in ClinicalTrials.gov by Sponsor Type, 2000–2019. JAMA Netw. Open. 2020;3:e2014682. doi: 10.1001/jamanetworkopen.2020.14682. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

