Acquisition of New Migratory Properties by Highly Differentiated CD4+CD28null T Lymphocytes in Rheumatoid Arthritis Disease
- PMID: 34202487
- PMCID: PMC8306508
- DOI: 10.3390/jpm11070594
Acquisition of New Migratory Properties by Highly Differentiated CD4+CD28null T Lymphocytes in Rheumatoid Arthritis Disease
Abstract
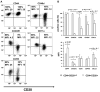
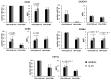
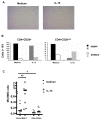
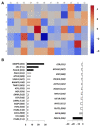
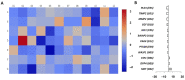
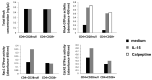
Expanded CD4+CD28null T lymphocytes are found in the tissues and peripheral blood of patients with many autoimmune diseases, such as rheumatoid arthritis (RA). These highly differentiated cells present potent inflammatory activity and capability to induce tissue destruction, which has been suggested to predispose to the development of more aggressive disease. In fact, preferential migration to inflammatory sites has been proposed to be a contributing factor in the progression of autoimmune and cardiovascular diseases frequently found in these patients. The functional activity of CD4+CD28null T lymphocytes is largely dependent on interleukin 15 (IL-15), and this cytokine may also act as a selective attractor of these cells to local inflammatory infiltrates in damaged tissues. We have analysed, in RA patients, the migratory properties and transcriptional motility profile of CD4+CD28null T lymphocytes compared to their counterparts CD28+ T lymphocytes and the enhancing role of IL-15. Identification of the pathways involved in this process will allow us to design strategies directed to block effector functions that CD4+CD28null T lymphocytes have in the target tissue, which may represent therapeutic approaches in this immune disorder.
Keywords: CD4+CD28null T lymphocytes; IL-15; migration; rheumatoid arthritis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
CD4+CD28- T lymphocytes contribute to early atherosclerotic damage in rheumatoid arthritis patients.Circulation. 2004 Jun 8;109(22):2744-8. doi: 10.1161/01.CIR.0000131450.66017.B3. Epub 2004 May 24. Circulation. 2004. PMID: 15159291
-
CD4+CD28null T cells in autoimmune disease: pathogenic features and decreased susceptibility to immunoregulation.J Immunol. 2007 Nov 15;179(10):6514-23. doi: 10.4049/jimmunol.179.10.6514. J Immunol. 2007. PMID: 17982040
-
Expansion of CD4+CD28null T-lymphocytes in diabetic patients: exploring new pathogenetic mechanisms of increased cardiovascular risk in diabetes mellitus.Eur Heart J. 2011 May;32(10):1214-26. doi: 10.1093/eurheartj/ehq499. Epub 2011 Jan 8. Eur Heart J. 2011. PMID: 21217142
-
CD28 null CD4 T-cell expansions in autoimmune disease suggest a link with cytomegalovirus infection.F1000Res. 2019 Mar 25;8:F1000 Faculty Rev-327. doi: 10.12688/f1000research.17119.1. eCollection 2019. F1000Res. 2019. PMID: 30984377 Free PMC article. Review.
-
Expansions of Cytotoxic CD4+CD28- T Cells Drive Excess Cardiovascular Mortality in Rheumatoid Arthritis and Other Chronic Inflammatory Conditions and Are Triggered by CMV Infection.Front Immunol. 2017 Mar 2;8:195. doi: 10.3389/fimmu.2017.00195. eCollection 2017. Front Immunol. 2017. PMID: 28303136 Free PMC article. Review.
Cited by
-
Are immunosenescent T cells really senescent?Aging Cell. 2024 Oct;23(10):e14300. doi: 10.1111/acel.14300. Epub 2024 Aug 7. Aging Cell. 2024. PMID: 39113243 Free PMC article. Review.
-
Activation of senescence in critically ill patients: mechanisms, consequences and therapeutic opportunities.Ann Intensive Care. 2024 Jan 5;14(1):2. doi: 10.1186/s13613-023-01236-4. Ann Intensive Care. 2024. PMID: 38180573 Free PMC article. Review.
-
Personalized Medicine in Autoimmune Diseases.J Pers Med. 2021 Nov 11;11(11):1181. doi: 10.3390/jpm11111181. J Pers Med. 2021. PMID: 34834532 Free PMC article.
References
-
- Ogawa S., Abe R. Advances in Experimental Medicine and Biology. Volume 1189. Springer; Berlin/Heidelberg, Germany: 2019. Signal transduction via co-stimulatory and co-inhibitory receptors; pp. 85–133. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

