Systems Biology and Bile Acid Signalling in Microbiome-Host Interactions in the Cystic Fibrosis Lung
- PMID: 34202495
- PMCID: PMC8300688
- DOI: 10.3390/antibiotics10070766
Systems Biology and Bile Acid Signalling in Microbiome-Host Interactions in the Cystic Fibrosis Lung
Abstract
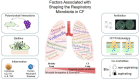
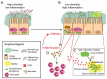
The study of the respiratory microbiota has revealed that the lungs of healthy and diseased individuals harbour distinct microbial communities. Imbalances in these communities can contribute to the pathogenesis of lung disease. How these imbalances occur and establish is largely unknown. This review is focused on the genetically inherited condition of Cystic Fibrosis (CF). Understanding the microbial and host-related factors that govern the establishment of chronic CF lung inflammation and pathogen colonisation is essential. Specifically, dissecting the interplay in the inflammation-pathogen-host axis. Bile acids are important host derived and microbially modified signal molecules that have been detected in CF lungs. These bile acids are associated with inflammation and restructuring of the lung microbiota linked to chronicity. This community remodelling involves a switch in the lung microbiota from a high biodiversity/low pathogen state to a low biodiversity/pathogen-dominated state. Bile acids are particularly associated with the dominance of Proteobacterial pathogens. The ability of bile acids to impact directly on both the lung microbiota and the host response offers a unifying principle underpinning the pathogenesis of CF. The modulating role of bile acids in lung microbiota dysbiosis and inflammation could offer new potential targets for designing innovative therapeutic approaches for respiratory disease.
Keywords: Pseudomonas aeruginosa; aspiration; bile acids; chronic infection; cystic fibrosis; gastro-oesophageal reflux; inflammation; lung; microbiota; pathogen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- WHO (World Health Organisation) Global Status Report on Noncommunicable Diseases 2010. WHO; Geneva, Switzerland: 2011.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

