Exogenous Stilbenes Improved Tolerance of Arabidopsis thaliana to a Shock of Ultraviolet B Radiation
- PMID: 34202535
- PMCID: PMC8308955
- DOI: 10.3390/plants10071282
Exogenous Stilbenes Improved Tolerance of Arabidopsis thaliana to a Shock of Ultraviolet B Radiation
Abstract
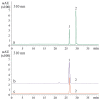
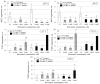
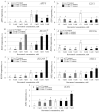
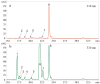
Excessive ultraviolet B (UV-B) irradiation is one of the most serious threats leading to severe crop production losses. It is known that secondary metabolite biosynthesis plays an important role in plant defense and forms a protective shield against excessive UV-B irradiation. The contents of stilbenes and other plant phenolics are known to sharply increase after UV-B irradiation, but there is little direct evidence for the involvement of stilbenes and other plant phenolics in plant UV-B protection. This study showed that foliar application of trans-resveratrol (1 and 5 mM) and trans-piceid (5 mM) considerably increased tolerance to a shock of UV-B (10 min at 1800 µW cm-2 of irradiation intensity) of four-week-old Arabidopsis thaliana plants that are naturally incapable of stilbene production. Application of trans-resveratrol and trans-piceid increased the leaf survival rates by 1-2%. This stilbene-induced improvement in UV-B tolerance was higher than after foliar application of the stilbene precursors, p-coumaric and trans-cinnamic acids (only 1-3%), but less than that after treatment with octocrylene (19-24%), a widely used UV-B absorber. Plant treatment with trans-resveratrol increased expression of antioxidant and stress-inducible genes in A.thaliana plants and decreased expression of DNA repair genes. This study directly demonstrates an important positive role of stilbenes in plant tolerance to excessive UV-B irradiation, and offers a new approach for plant UV-B protection.
Keywords: UV-B irradiation; piceid; plant UV tolerance; resveratrol; stilbenes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Kakani V.G., Reddy K.R., Zhao D., Sailaja K. Field crop responses to ultraviolet-B radiation: A review. Agric. For. Meteorol. 2003;120:191–218. doi: 10.1016/j.agrformet.2003.08.015. - DOI
-
- Zuk-Golaszewska K., Upadhyaya M.K., Golaszewski J. The effect of UV-B radiation on plant growth and development. Plant Soil Environ. 2003;49:135–140. doi: 10.17221/4103-PSE. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

