Combining Augmented Radiotherapy and Immunotherapy through a Nano-Gold and Bacterial Outer-Membrane Vesicle Complex for the Treatment of Glioblastoma
- PMID: 34202555
- PMCID: PMC8306693
- DOI: 10.3390/nano11071661
Combining Augmented Radiotherapy and Immunotherapy through a Nano-Gold and Bacterial Outer-Membrane Vesicle Complex for the Treatment of Glioblastoma
Abstract
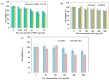
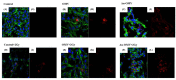
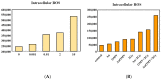
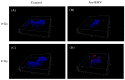
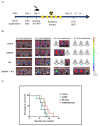
Glioblastoma, formerly known as glioblastoma multiforme (GBM), is refractory to existing adjuvant chemotherapy and radiotherapy. We successfully synthesized a complex, Au-OMV, with two specific nanoparticles: gold nanoparticles (AuNPs) and outer-membrane vesicles (OMVs) from E. coli. Au-OMV, when combined with radiotherapy, produced radiosensitizing and immuno-modulatory effects that successfully suppressed tumor growth in both subcutaneous G261 tumor-bearing and in situ (brain) tumor-bearing C57BL/6 mice. Longer survival was also noted with in situ tumor-bearing mice treated with Au-OMV and radiotherapy. The mechanisms for the successful treatment were evaluated. Intracellular reactive oxygen species (ROS) greatly increased in response to Au-OMV in combination with radiotherapy in G261 glioma cells. Furthermore, with a co-culture of G261 glioma cells and RAW 264.7 macrophages, we found that GL261 cell viability was related to chemotaxis of macrophages and TNF-α production.
Keywords: glioblastoma; gold nanoparticles; immunotherapy; outer membrane vesicles; radioenhancer.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Schuemann J., Bagley A.F., Berbeco R., Bromma K., Butterworth K.T., Byrne H.L., Chithrani B.D., Cho S.H., Cook J.R., Favaudon V., et al. Roadmap for metal nanoparticles in radiation therapy: Current status, translational challenges, and future directions. Phys. Med. Biol. 2020;65:21RM02. doi: 10.1088/1361-6560/ab9159. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

