Perfusable System Using Porous Collagen Gel Scaffold Actively Provides Fresh Culture Media to a Cultured 3D Tissue
- PMID: 34202572
- PMCID: PMC8269041
- DOI: 10.3390/ijms22136780
Perfusable System Using Porous Collagen Gel Scaffold Actively Provides Fresh Culture Media to a Cultured 3D Tissue
Abstract
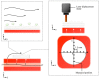
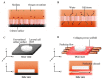
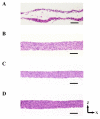
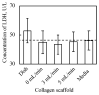
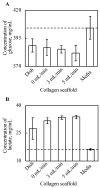
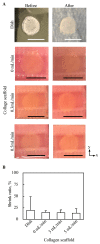
Culturing three-dimensional (3D) tissues with an appropriate microenvironment is a critical and fundamental technology in broad areas of cutting-edge bioengineering research. In addition, many technologies have engineered tissue functions. However, an effective system for transporting nutrients, waste, or oxygen to affect the functions of cell tissues has not been reported. In this study, we introduce a novel system that employs diffusion and convection to enhance transportation. To demonstrate the concept of the proposed system, three layers of normal human dermal fibroblast cell sheets are used as a model tissue, which is cultured on a general dish or porous collagen scaffold with perfusable channels for three days with and without the perfusion of culture media in the scaffold. The results show that the viability of the cell tissue was improved by the developed system. Furthermore, glucose consumption, lactate production, and oxygen transport to the tissues were increased, which might improve the viability of tissues. However, mechanical stress in the proposed system did not cause damage or unintentional functional changes in the cultured tissue. We believe that the introduced culturing system potentially suggests a novel standard for 3D cell cultures.
Keywords: 3D tissue culture; cell sheet technology; convection-diffusion equation; perfusion system.
Conflict of interest statement
T.S. was a shareholder of CellSeed, Inc., Tokyo, Japan. Tokyo Women’s Medical University receives research funds from CellSeed, Inc. Tokyo Women’s Medical University and Waseda University receives research funds from CKD Corporation.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

