Metabolite and Elastase Activity Changes in Beach Rose (Rosa rugosa) Fruit and Seeds at Various Stages of Ripeness
- PMID: 34202618
- PMCID: PMC8309187
- DOI: 10.3390/plants10071283
Metabolite and Elastase Activity Changes in Beach Rose (Rosa rugosa) Fruit and Seeds at Various Stages of Ripeness
Abstract
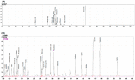
Rose hips are the fruits of the beach rose (Rosa rugosa). To determine the optimal harvest time and to obtain the maximum functional compounds, rose hips at various stages of ripeness (immature, early, mid, and late) were harvested, and the flesh tissue and seeds were separated. The rose hip flesh showed the highest total phenolic content at the mid-ripeness stage (8.45 ± 0.62 mg/g gallic acid equivalent concentration (dry weight)). The early-, mid-, and late-ripeness stages of rose hip flesh did not show significantly different 2,2-diphenyl-1-picrylhydrazyl antioxidant capacities. The elastase inhibitory activity of the 95% ethanol extract from the rose hip seeds was highest at the mid-ripeness stage; however, the elastase inhibitory activity of the rose hip tissue was not significantly different from that of the seeds. Pathway analysis using MetaboAnalyst showed that sucrose, fructose, and glucose gradually increased as the fruit ripened. Ursolic acid was detected in the seeds but not in the flesh. Of the fatty acids, linoleic acid concentrations were highest in rose hip seeds, followed by linolenic acid, oleic acid, and palmitic acid. Fatty acids and ursolic acid might be the active compounds responsible for elastase inhibitory activity and can be utilized as a functional cosmetic material.
Keywords: Rosa rugosa; antioxidant; elastase inhibitory activity; fatty acid; primary metabolite; ripening stages; rose hip.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Dubey S.P., Lahtinen M., Sillanpää M. Green Synthesis and Characterizations of Silver and Gold Nanoparticles Using Leaf Extract of Rosa Rugosa. Colloids Surf. A Physicochem. Eng. Asp. 2010;364:34–41. doi: 10.1016/j.colsurfa.2010.04.023. - DOI
-
- Christenhusz M.J.M., Byng J.W. The Number of Known Plants Species in the World and Its Annual Increase. Phytotaxa. 2016;261:201–217. doi: 10.11646/phytotaxa.261.3.1. - DOI
-
- Park B.-J. Isolation of Main Component and Antioxidant Activities on the Stem and Root of Rosa Rugosa. Korean J. Plant Resour. 2008;21:402–407.
Grants and funding
LinkOut - more resources
Full Text Sources

