Quaternary Ammonium Compounds (QACs) and Ionic Liquids (ILs) as Biocides: From Simple Antiseptics to Tunable Antimicrobials
- PMID: 34202677
- PMCID: PMC8268321
- DOI: 10.3390/ijms22136793
Quaternary Ammonium Compounds (QACs) and Ionic Liquids (ILs) as Biocides: From Simple Antiseptics to Tunable Antimicrobials
Abstract
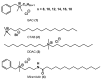
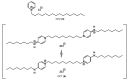
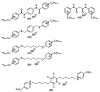
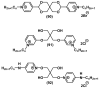
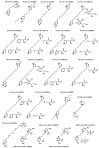
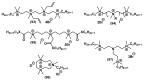
Quaternary ammonium compounds (QACs) belong to a well-known class of cationic biocides with a broad spectrum of antimicrobial activity. They are used as essential components in surfactants, personal hygiene products, cosmetics, softeners, dyes, biological dyes, antiseptics, and disinfectants. Simple but varied in their structure, QACs are divided into several subclasses: Mono-, bis-, multi-, and poly-derivatives. Since the beginning of the 20th century, a significant amount of work has been dedicated to the advancement of this class of biocides. Thus, more than 700 articles on QACs were published only in 2020, according to the modern literature. The structural variability and diverse biological activity of ionic liquids (ILs) make them highly prospective for developing new types of biocides. QACs and ILs bear a common key element in the molecular structure-quaternary positively charged nitrogen atoms within a cyclic or acyclic structural framework. The state-of-the-art research level and paramount demand in modern society recall the rapid development of a new generation of tunable antimicrobials. This review focuses on the main QACs exhibiting antimicrobial and antifungal properties, commercial products based on QACs, and the latest discoveries in QACs and ILs connected with biocide development.
Keywords: antibacterial; antimicrobial; biocide; ionic liquid; quaternary ammonium compound.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

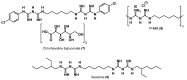
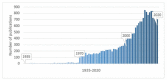
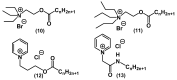
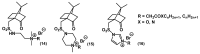
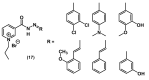

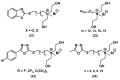
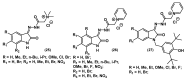
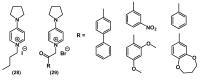
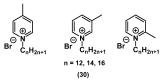
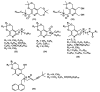
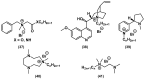

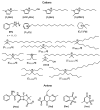
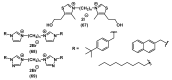
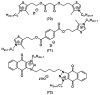
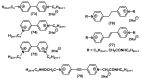
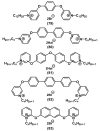
References
-
- Paulson D.S. New Biocides Development. Volume 967. American Chemical Society; Washington, DC, USA: 2007. Topical Antimicrobials; pp. 124–150.
-
- Jacobs W.A., Heidelberger M., Amoss H.L. The Bactericidal Properties of The Quaternary Salts of Hexamethylenetetramine: II. The Relation Between Constitution and Bactericidal Action in the Substituted Benzylhexamethylenetetraminium. Salts. J. Exp. Med. 1916;23:569–576. doi: 10.1084/jem.23.5.569. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

